| Review Article, Biomed Biopharm Res., 2023; 20(1):106-125 doi: 10.19277/bbr.20.1.307; PDF version [+]; Portuguese html [PT] |
Methods for cutaneous penetration assessment of organic UV filters - a review
Débora Quintas Balla 1 ![]() , Alicio Vitorino de Souza Neto 1
, Alicio Vitorino de Souza Neto 1 ![]() , Renata Miliani Martinez 2
, Renata Miliani Martinez 2 ![]() & Alexandra de Almeida Hübner 2
& Alexandra de Almeida Hübner 2 ![]() , Catarina Rosado 3
, Catarina Rosado 3 ![]() , André Rolim Baby 2
, André Rolim Baby 2 ![]() , Fabiana Vieira Lima1
, Fabiana Vieira Lima1 ![]() ✉️
✉️
1 - Laboratory of Pharmaceutical Technology, Department of Health Science, Federal University of Espírito Santo, São Mateus, ES, Brazil
2 - Department of Pharmacy, Faculty of Pharmaceutical Sciences, University of São Paulo, São Paulo, SP, Brazil
3 - CBIOS - Center for Biosciences & Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisboa, Portugal
Abstract
Sunscreens are currently considered cosmetic assets and their use has considerably increased since consumers have become greatly aware of the damages induced by ultraviolet (UV) radiation on the skin, such as premature aging and cancer. However, concerns have arisen over the percutaneous absorption of UV filters. In order to ensure both efficacy and safety, sunscreens need to remain in the outermost layers of the stratum corneum, because their penetration into the dermis can cause systemic effects. Herein, a review was conducted of specialized literature published between 2000 to 2020, clustering studies focused on the skin penetration of UV filters. In this context, different in vitro and in vivo methodologies employed to assess the penetration of such compounds are highlighted, such as those based on tape stripping and diffusion cells. When combined with analytical methods, such as high-performance liquid chromatography, it is possible to trace a profile of the penetration of UV filters and elucidate factors that interfere with this phenomenon. Moreover, studies have been carried out on dissemination strategies that aim to encapsulate the molecules of UV filters, and/or change their physicochemical characteristics, in effort to increase the efficacy and safety of these formulations.
Keywords: Franz cell, sunscreens, percutaneous penetration, tape stripping
To Cite: Quintas Balla, D., Vitorino de Souza Neto, A., Miliani Martinez, R., de Almeida Hübner, A., Rosado, C., Rolim Baby, A., Vieira Lima, F. (2023) Methods for cutaneous penetration assessment of organic UV filters - a review. Biomedical and Biopharmaceutical Research, 20(1), 106-125.
Correspondence to:
Received 03/04/2023; Accepted 31/05/2023
Introduction
The skin is the largest human organ in dimension and of relevance for the application of pharmacological and cosmetic substances. In this context, studies on the behavior of chemical actives when applied on the skin and on the mechanisms regulating their penetration through the stratum corneum (SC) are clearly important. SC acts on the skin as a diffusion barrier and, thus, several physicochemical characteristics of the components of the applied formulation should be considered for this analysis (1).
Sunscreens are among the most commonly used formulations for the skin, especially as exposure to ultraviolet radiation (UVR) is associated with various harmful effects such as skin cancer, erythema and premature skin aging (2). Sunscreens are defined by regulatory agencies as substances with the exclusive and/or main purpose of protecting the skin against UVR by absorbing, dispersing or reflecting radiation (3,4).
UV filters can be classified according to their mechanism of action as chemical (or organic) or as physical (or inorganic). To be effective, chemical filters need to accumulate in the SC layers, as they act by absorbing the UVR. The physical filters, in turn, must remain on the SC surface, forming a film reflecting the UVR (5).
When these molecules penetrate the stratum corneum, they can permeate through the remaining skin layers, leading to systemic circulation. The SC is the primary barrier to the penetration of substances, and can also act as a reservoir of these substances that are applied to the skin (6).
The penetration mechanism involves the entry of the sunscreen molecule into the most superficial layer of the skin but does not require the passage of it from one layer to another as occurs in the permeation movement (7). As it influences the partition from the formulation and its diffusion in the skin, the lipophilicity of the active molecules is one of the most significant mechanisms facilitating this permeation, and it is an important property to be studied to understand the penetration of sunscreens in the SC (8).
Penetration is the first step for filters to reach the vascularized layers of the skin, which can trigger toxic effects (9). Moreover, the permeation of the filters can lead to the loss of photoprotection, because, as mentioned above, the photoprotective action depends on the molecules remaining in the most superficial layers of the SC (10,11).
In this framework, several methodologies for the evaluation of the penetration and permeation of substances in SC have been developed. Thus, the objective of this work was to conduct a review of the literature regarding the different in vitro and in vivo methodologies employed in the study of the penetration of chemical UV filters in the skin, as well as the factors that can influence the penetration profile of sunscreens in the skin.
Material and Methods
The bases used for search were Google Scholar, Scielo, Pubmed, Scopus and CAPES Journals. The keywords used for the research were "sunscreen" and "penetration". The publication period selected for the research comprised publications from the years 2000 to 2020, finding a total of 20,277 results. Based on the results found, a selection was made by title, where 398 results were selected. The exclusion criteria of the titles used were the absence of the searched terms, the absence of methods that assess the penetration of filters, absence of chemical filters and, finally, review papers. The Mendeley Desktop platform was used to perform the selection, reading of abstracts and exclude duplicates. After reading the abstracts, 122 articles were selected. The same exclusion criteria were used after reading the articles, resulting in the selection of 61 articles for this review.
Results and Discussion
The articles were analyzed in relation to the methodology used to evaluate the penetration and/or permeation of sunscreens on the skin, regarding the substrate used as a model for analysis and for the results presented.
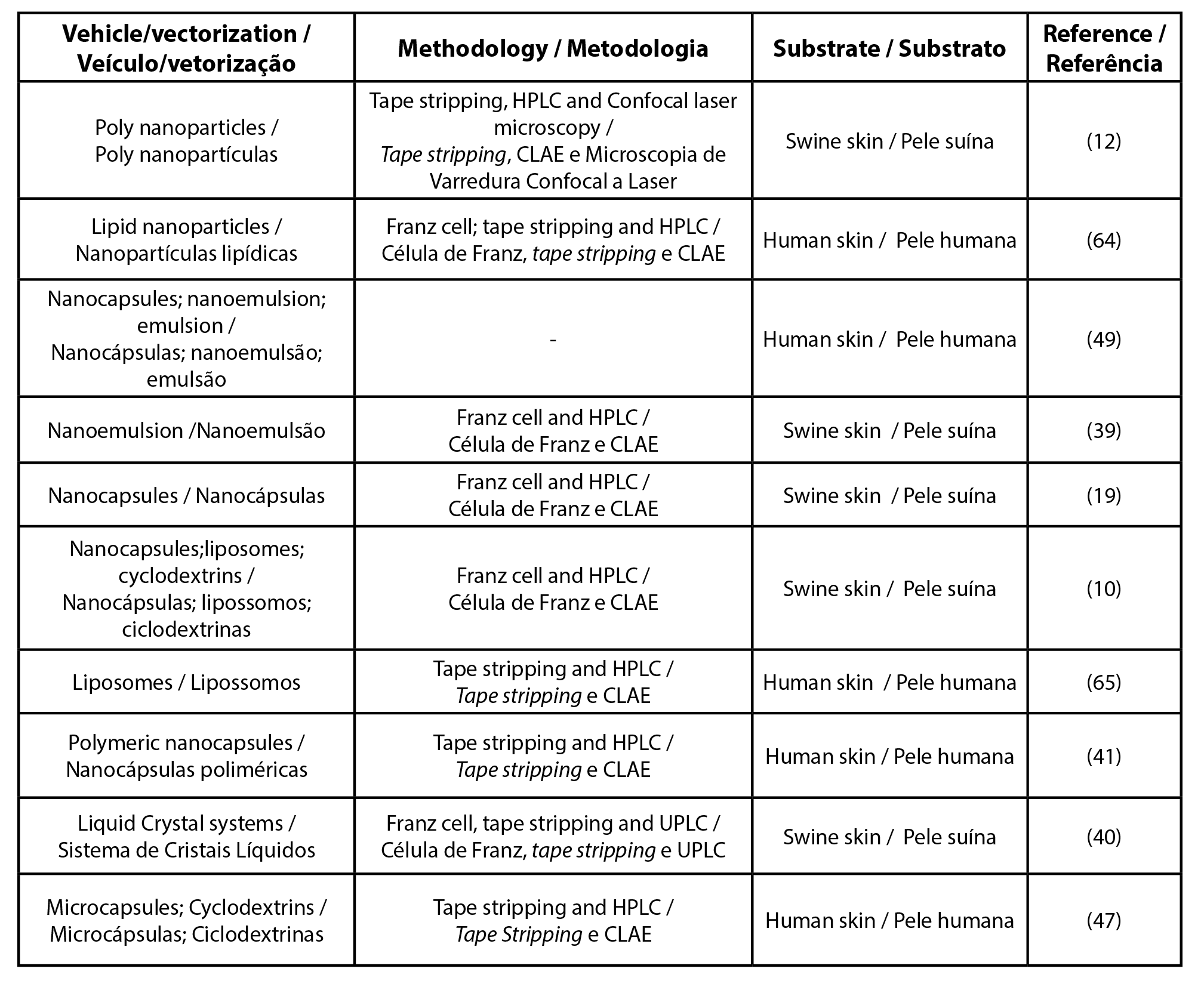
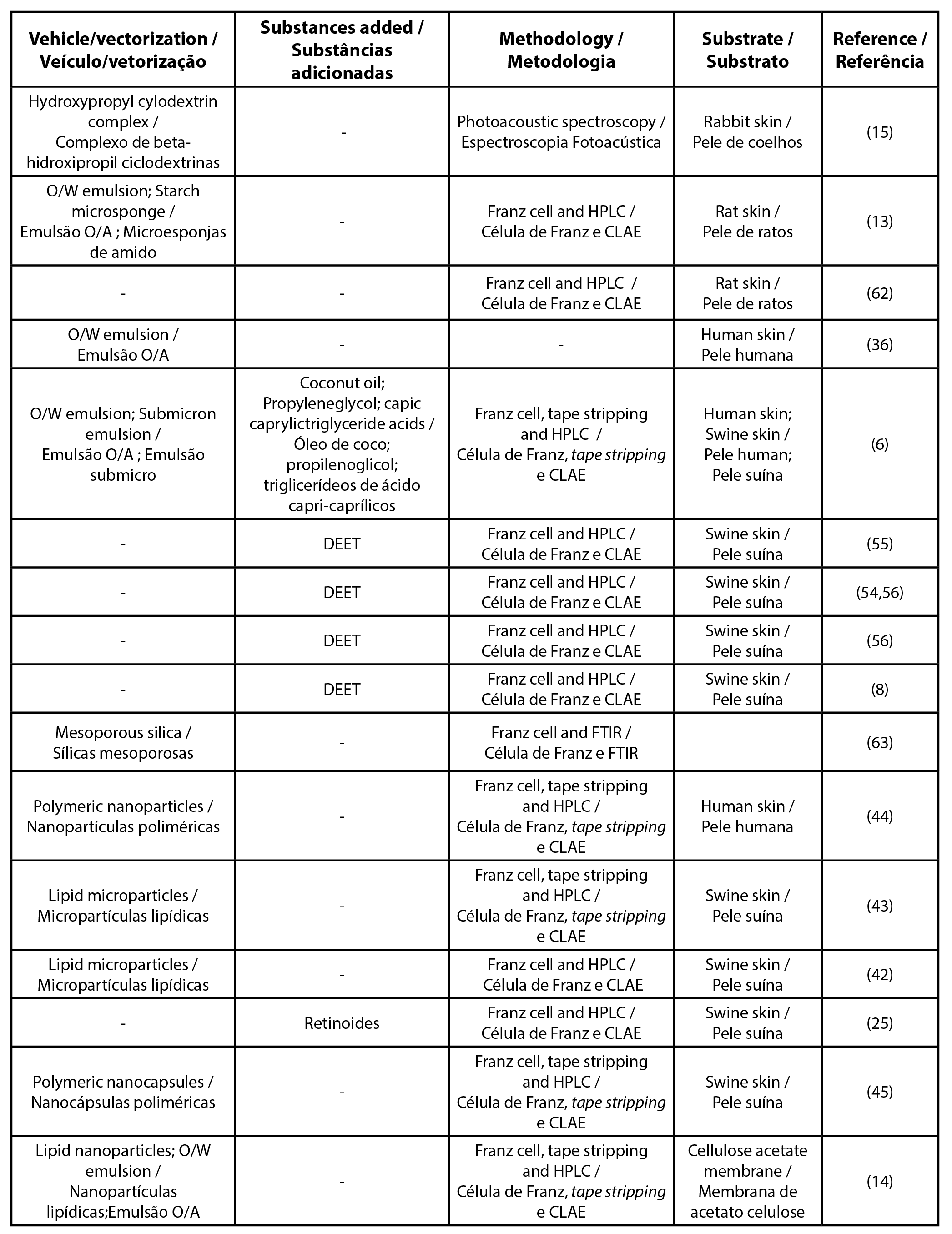
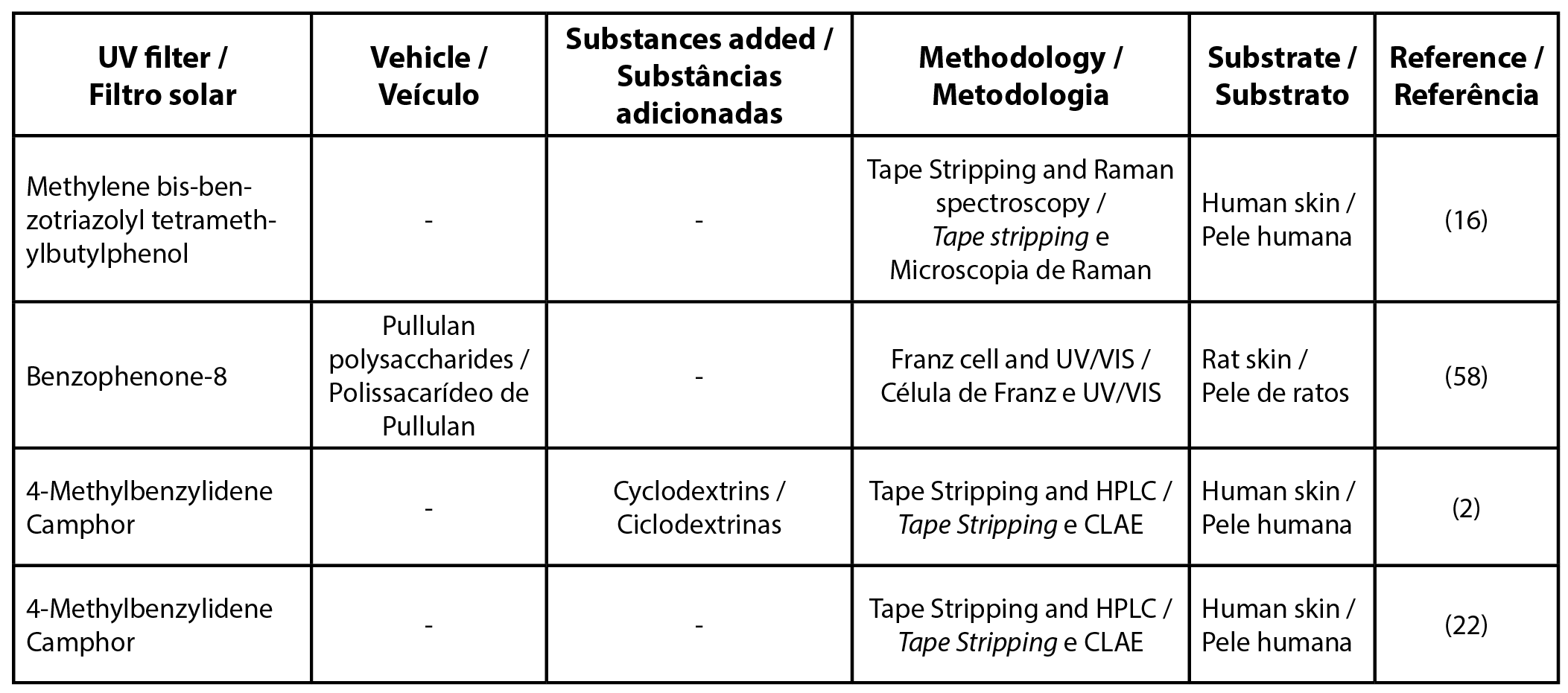
To unify the UV filter nomenclature the international nomenclature of cosmetic ingredients (INCI) was used, other synonyms and details can be found in the Supplementary Table. Among the most cited filters found in this review were butyl methoxydibenzoylmethane, ethylhexyl methoxycinnamate, and benzophenone-3 (Tables 1, 2 and 3, respectively). Table 4 details the list of UV filters found in isolation, whereas Table 5 contains filters in association, as well as the vehicles used, the methodology employed and the substrate that was used as a study model.
| Table 1 - List of articles for UV filter butyl methoxydibenzoylmethane. |
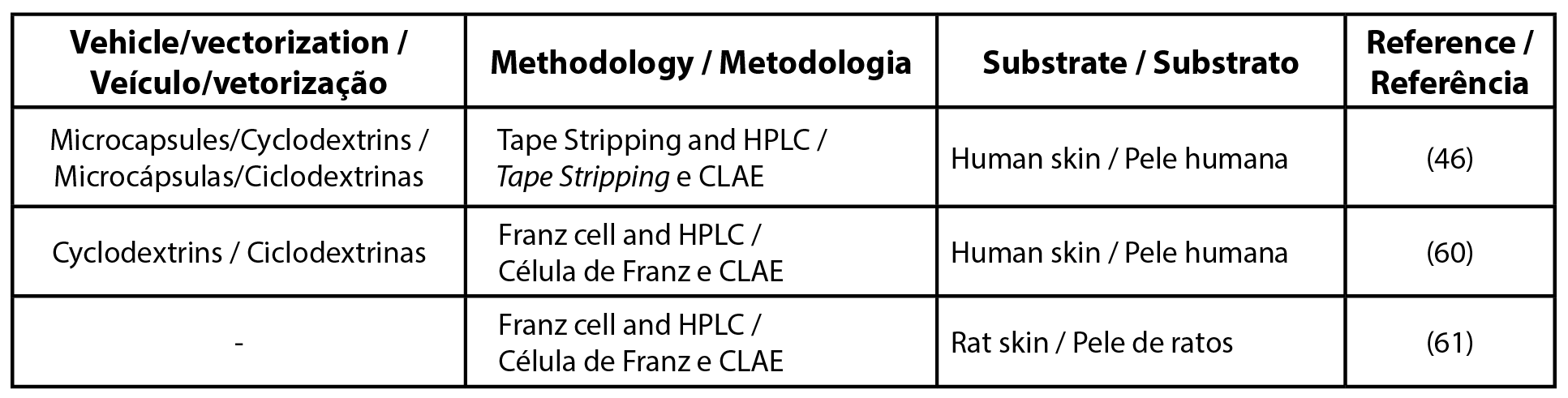 |
| Legend: HPLC - high-performance liquid chromatography |
| Table 2 - List of articles for UV filter ethylhexyl methoxycinnamate. |
 |
| Legend: HPLC - high-performance liquid chromatography ; UPLC - ultra high performance liquid chromatography |
| Table 3 - List of articles for UV filter benzophenone-3. |
 |
| Legend: HPLC - high-performance liquid chromatography; O/W - oil/water; W/O - water/oil; DEET - N, N-diethyl-m-toluamide |
| Table 4 - List of articles by UV filter used alone. |
 |
| Legend: HPLC - high-performance liquid chromatography |
| Table 5 - List of articles for UV filters in association |
 |
| Legend: HPLC - high-performance liquid chromatography ; UPLC - ultra high performance liquid chromatography |
Part of the studies selected in this review were conducted on formulations that could increase the retention of filters in the SC, avoiding their permeation to the dermis and hypodermis by encapsulation with the use of nano and microparticles, for example, and will be highlighted below.
The tape stripping technique and the study of diffusion using Franz diffusion cells were the most common approaches found among the studies. Some authors chose to use both methodologies in the same study in order to evaluate penetration in vivo and in vitro. Nevertheless, other methodologies were found, such as those based on photoacoustic microscopic mapping using Raman and electron microscopy (12-17).
The substrate used as a model membrane also varied, such as rat skin (18), rabbit (15), pig (19), and human skin (20), when performing the in vitro study.
Tape stripping
The evaluation of the penetration of UV filters in the skin is relevant for determining the safety of those used in sunscreen formulations. Studies have been developed to improve techniques that make it possible to trace the penetration profile of these substances, among them, the tape stripping technique has been widely used, being one of the most common methods for evaluating the penetration of substances in the SC.
This methodology is performed by applying and removing adhesive tapes successively in an area of the skin, which needs to be clean and free of hair. If these tapes are applied successively in the same skin site, they can contain an entire layer of SC, with the first tape containing the most superficial layer of cells and the other tapes corresponding to deeper layers (21).
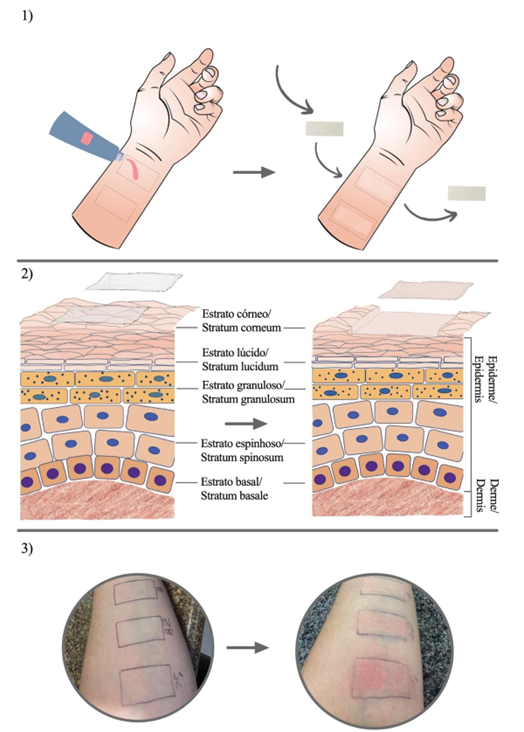
The aggregates of corneocytes along with the substances that have been topically applied are transferred to the tapes, after which they are analyzed by quantitative spectrophotometric methods. One of the most commonly employed methods is UV/VIS spectrophotometry (22). Figure 1 shows the step by step application of the method on a volunteer's forearm.
| Figure 1 - Representation of the tape stripping method: 1) The area is demarcated and specific quantity of the product is applied, and after a set time the tapes are applied and removed successively from the same location; 2) Representation of skin layers and superficial layers of the stratum corneum removed by the tapes; 3) Photographs demonstrating the procedure before and after the removal of the tapes at the place of application. |
 |
One benefit of the tape stripping technique is that it is non-invasive and can be employed in vivo studies (6,16), which makes the study more realistic simulating the conditions of use of the formulation (23).
This methodology can also be applied in vitro, when used in conjunction with Franz cell, in which the SC of the biological model being studied is removed with adhesive tapes, making it possible to trace the penetration profile in the SC and the permeation profile of the substances at the same time. The membrane models used in these cases can be human skin discarded after plastic surgeries or animal models such as pig skin (24,25).
Some studies which used this methodology to evaluate the penetration of filters have shown that most of the filters applied to the skin could be recovered in the first tapes. This means that they penetrated only the most superficial layers of the SC, without evidence that deep penetration can be considered to the point of finding systemic circulation. It was also found that this penetration depends on the level of lipophilicity of the molecule, presenting greater affinity for SC, as is the case of benzophenone-3 (24,26).
Another benefit of using tape stripping is that it can evaluate penetration in a short period of time and with a small amount of filter applied to the skin (27). However, Couteau et al. (2001) demonstrated that when evaluating penetration over a longer period of time, the recovery content of the filters decreases, leading to the conclusion that the longer the filters remain on the skin, the greater their penetration (28).
When making a comparison between the techniques used to evaluate the penetration of sunscreens on the skin, tape stripping for an in vivo study and Franz cell for an in vitro analysis, researchers realized that both techniques, when used together, could help in the creation of new formulations of sunscreens, since the in vivo study model provides information on the penetration of filters in SC and the in vitro study model can help in the screening of new molecules that can act as sunscreens and new vehicles for formulations (14).
The tape stripping technique can also be combined with other analytical methods such as Raman confocal microscopy, that allows visualization of the contents of the tapes obtaining a three-dimensional view of the distribution of filters in the SC (16). It was also used in conjunction with spectrometric methods, such as photoacoustic spectroscopy, which enables evaluating the depth of the penetration profile of the filters through the length of thermal diffusion (15). Gebauer et al. (2012) demonstrated that this technique tied to tape stripping is useful in the analysis of the homogeneity of the UV filter distribution in the SC (29). However, Haque et al. (2016) observed that some excipients used in the formulation may interfere with the adhesive strip (30).
Regarding the results found on the recovery of filters in studies that used the tape stripping technique, it is noted that most have a recovery content of about 90%, as demonstrated for methylene bis-benzotriazolyl tetramethylbutylphenol - 91.1%, ethylhexyl methoxycinnamate – 103.5%, for diethylamino hydroxybenzoyl hexyl benzoate - 101.1%; for bis-ethylhexyloxyphenol methoxyphenyl triazine - 90.6% (16,31).
Franz diffusion cells
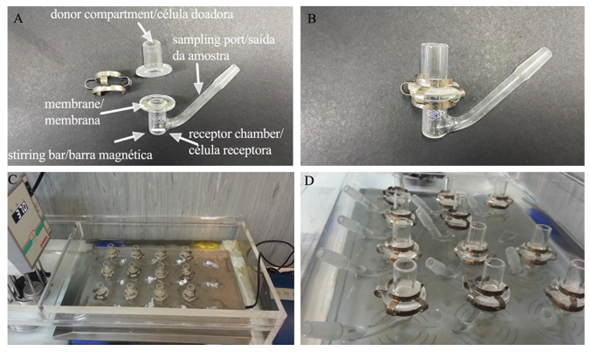
This procedure is very often employed in the study of the skin permeation of topically applied substances. A Franz diffusion cell consists of two compartments, a recipient and a donor. The filter to be studied is deposited in the donor compartment, while in the receiving compartment there is a medium into which the compound diffuses, with a magnetic stirrer maintaining its homogeneity (32).
The two compartments are separated by a model membrane. Diverse tissues can be used, such as human skin or relevant animal models such as pigs, rats or rabbits (31-33). The dermis side of the cutaneous model is positioned next to the receptor compartment and the SC side faces the donor, as shown in Figure 2. With Franz diffusion cells, it is possible to predict whether there will be systemic permeation of the filters, but the model membrane used should be considered in order to avoid bias, since it has been found that pig skin has greater permeability than human skin (30).
| Figure 2 - Representation of the Franz cell diffusion system: A) Parts of the cell; B) Cell arranged for use; C and D) Cells in a 37oC water bath. |
 |
Many of the studies found in this review used the Franz cell as a research source for substance permeation in the skin in combination with the tape stripping technique, using analytical methods to quantify the penetration and permeation of substances with an in vivo and in vitro research model (6).
Study model membranes
As observed in the previous section, it is possible to perform penetration and permeation studies with several model membranes. In this review, synthetic membranes as well as human, pig, rats and rabbits skin were found (Tables 1-5).
The use of human skin is the most indicated for the study of sunscreen penetration because that is where the product is intended. The studies can be performed in vivo when performed on volunteers, or in vitro using skin removed from plastic surgeries, usually from the abdominal region or breast region (20).
When pig skin is used, many researchers prefer to use that removed from the ear, being considered an adequate study model since it is very similar to human skin (34). However, it has been found that when using the tape stripping method, it is possible to remove greater amounts of SC when this membrane is employed (30).
Factors that determine the diffusion of sunscreens on the skin
Among the factors that determine the diffusion of sunscreens in the skin are: physicochemical characteristics, vehicle used in the formulation, concentration of molecules and skin status (31). The first two of these factors will be highlighted below.
An important feature to be observed is the lipophilicity of the molecule. Lipophilic filters such as benzophenone-3, ethylhexyl methoxycinnamate, or 2-ethylhexyl salicylate are easier to penetrate the SC (27,35). The skin has both hydrophilic and lipophilic areas, and the partition coefficient (expressed as log P) can be used to predict the penetration of these molecules. If the partition coefficient is too low, the molecules are more water soluble and will have difficulties crossing the hydrophobic barrier of the stratum corneum (the lipophilic part of the skin). On the other hand, if lipophilicity increases too much, the molecules will remain in the SC and will have a low partition into the viable epidermis which is hydrophilic. The previous study showed the percutaneous penetration presented predicted behaviors according to log P, considering ideal log P 1 (31).
Vehicle and vectorization
There are several ways to modulate the penetration of sunscreens, such as changing the physical and chemical properties of the formulation, including increasing the viscosity of the formulation (26,36).
It is consensually considered that the vehicle used can influence percutaneous absorption, either increasing or blocking the passage of the UV filters (26). Therefore, the composition of the formulation should be carefully chosen to prevent the penetration of sunscreens into the skin (37).
A study by Chatelain et al. (2003) demonstrated that filter penetration is dependent on the vehicle used in the formulation. In that study, the filters benzophenone-3, ethylhexyl methoxycinnamate, 2-ethylhexyl salicylate, homosalate and butyl methoxydibenzoylmethane had higher recovery rates when in formulations that had a gel cream as a vehicle rather than in petroleum jelly (38). In addition, the Sun Protection Factor (SPF) remained higher when the vehicle consisted of a gel cream, demonstrating that the filters remained in the most superficial layers of the SC. In another work, O/W emulsions have been shown to improve the penetration of benzophenone-4 (28).
Encapsulating the active molecules of the sunscreen has been shown to be a useful formulation strategy to increase the stability and efficacy of products containing UV filters (33). It has been shown that nanoemulsions can also assist in maintaining filters in the uppermost layers of SC (39).
Other studies have revealed that the use of liquid crystal systems may decrease the permeability of ethylhexyl methoxycinnamate in the skin (40). The use of nanocapsules can reduce the penetration of ethylhexyl methoxycinnamate forming a film on the surface and reducing penetration into the SC (19,33) . On the other hand, the size of these nanoparticles should be taken into account, because particles larger than 10 μm remain on the surface of the skin, whereas particles smaller than 3 μm are distributed by the hair follicles and the SC (41).
The use of solid lipid microspheres caused a decrease in the penetration of ethylhexyl triazone and benzophenone-3, and was suggested by Mestres et al. (2010) as the ideal vehicle for formulations containing these filters (23,42). Martins et al. (2014) also suggested the formulation of these microspheres with natural waxes, such as bee wax and carnauba (43). On the other hand, the use of polymeric nanostructures and starch microsponges reduced the permeation of benzophenone-3 in the skin, even leading to an increase in the SPF (13,44).
Siqueira et al. (2011) observed that nanocapsules containing chitosan enabled maintaining benzophenone-3 in the most superficial layers of SC, and that vehicles containing these nanocapsules can prevent systemic distribution (45). Other studies have shown that the use of beta cyclodextrin microcapsules, as well as the use of lipid microparticles incorporated with ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane filters, were able to reduce the penetration of butyl methoxydibenzoylmethane through SC, an important effect to ensure protection to UV rays and also limit potential toxic reactions by these filters (46–48).
In a study conducted by Calderilla-Fajardo et al. (2006), when ethylhexyl methoxycinnamate was loaded in nanocapsules and nanoemulsions, in addition to altering the penetration and the retention of the filters in the SC, the SPF of the formulation was increased (49, 50).
A study conducted by Wissing & Müller (2002) showed that solid lipid nanoparticles enabled benzophenone-3 to remain longer on the skin surface, maintaining its sun protection function (14).
Influence of other ingredients
The use of adjuvants in formulations may or may not help the penetration of filters into the skin. Fernandez et al. (2000) showed that using coconut oil and triglycerides of capric and caprylic acid as solvents for UV filters limited skin permeation (6).
Another study showed that adding Transcutol® CG (diethylene glycol monoethyl ether) to transdermal bases facilitated the accumulation of benzophenone-3 and ethylhexyl methoxycinnamate filters in SC (51).
Some sunscreens can act as penetration enhancers of other substances. Studies show that in six out of nine tested products [ethylhexyl methoxycinnamate, 2-ethylhexyl salicylate, benzophenone-3, benzophenone-4, ethylhexyl dimethyl PABA and homosalate] increased the penetration of dichlorophenoxyacetic acid (2.4-D), a herbicide used by farm workers (52,53).
Since there are formulations on the market that combine repellent and sun protection functions, some studies show that sunscreens can penetrate more into the skin when applied together with the use of biocides, such as benzophenone-3 which can penetrate about 89% more, when a repellent, DEET (N,N-diethyl-m-toluamide), is applied on it, presenting a synergistic effect (54–56). In other publications it was observed that the addition of antioxidants, such as trans-resveratrol and beta carotene, can also improve the performance of UV filters, increasing their SPF and decreasing their penetration into the SC (57).
Some sunscreens need to be stabilized in the formulation, such as 4-methylbenzilidene camphor, a filter that is currently in disuse. One of the forms of stabilization used is through cyclodextrin complexes, but Scalia et al. (2007) observed that these complexes do not influence the penetration profile of 4-methylbenzilidene camphor (2). Heo et al. (2018) added polysaccharides of pullulan into benzophenone-8 showing that this conjugated can increase the time that the filter is retained on the skin (58).
Finally, in a work observing the impact of caffeine in sunscreen formulations, an increase of approximately 25% in the anti-UVB protection was obtained, combined with good skin compatibility (59).
Conclusion
Through the review of the articles in this study it is possible to conclude that the use of only one methodology can be considered for the evaluation of the penetration of sunscreens, but the combination of techniques such as Franz diffusion cells and tape stripping can reveal the complete profile of permeation and penetration of sunscreens in the skin.
The tape stripping technique reveals the penetration profile of filters associated with analytical methods such as high-performance liquid chromatography and spectrophotometric methods. It is observed that the penetration of the filters into the SC does not necessarily imply that the filters had a transdermal passage. For this reason, the recovery content of the filters should be taken into account, and researchers should observe in which layers of the SC this content is higher, since a higher recovery content in the first tapes suggests the non-permeation of the UV filter.
To evaluate whether there is transdermal permeation, the method based in Franz diffusion cells is suitable, because it evaluates the passage of these substances through all the skin layers, both epidermis and dermis, being possible to evaluate whether the sunscreens could be available to reach blood circulation and act systemically.
Several factors can influence the penetration of sunscreens, such as the substrate used, the physicochemical characteristics, such as the partition coefficient and lipophilicity of the molecule, the vehicles used, interactions with other substances and the vectorization thereof.
It is noted that the use of these methodologies is important to ensure the safety and efficacy of these products, since filters such as benzophenone-3, ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane, for example, present several reports that suggest penetration into the skin, and are still widely used in various formulations of sunscreens.
Contributions of the Authors
Conceptualization, D.Q.B, A.V.S.N., F.V.L.; methodology, D.Q.B, F.V.L.; formal analysis, C.R., R.M.M., A.R.B.; research, D.Q.B, A.V.S.N., F.V.L.; writing, D.Q.B, R.M.M., A.A.H., C.R. original draft preparation, D.Q.B. ; writing and editing, D.Q.B., R.M.M., F.V.L. C.R.; supervision, F.V.L.; project administration, F.V.L.; acquisition of financing, D.Q.B.; F.V.L. All authors read and agreed with the published version of the manuscript.
Funding
The authors thank the Foundation for Research and Innovation Support of Espírito Santo (FAPES) for the scientific initiation grant.
Conflicts of Interest
The editors involved in this manuscript's authorship had no participation in the review or decision process. All authors have stated that there are no financial and/or personal relationships that could represent a potential conflict of interest.
References
1. Alves, N.C. (2015) Penetração de ativos na pele: revisão bibliográfica. Amazônia Science & Health, 3(4), 36–43. https://doi.org/10.18606/2318-1419/amazonia.sci.health.v3n4p36-43
2. Scalia, S., Tursilli, R., & Iannuccelli, V. (2007) Complexation of the sunscreen agent, 4-methylbenzylidene camphor with cyclodextrins: Effect on photostability and human stratum corneum penetration. Journal of Pharmaceutical and Biomedical Analysis, 44(1), 29–34. https://doi.org/10.1016/j.jpba.2007.01.016
3. BRASIL. (2012) Resolução. RDC no 30, de 1o de junho de 2012. Aprova o Regulamento Técnico Mercosul sobre Protetores solares em Cosméticos e dá outras providências, 1-7.
4. EUROPEAN UNION. (2009) Regulation (EC) no 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Official Journal of the European Union L, 342, 59-202.
5. Flor, J., Davolos, M.R., & Correa, M.A. (2007) Protetores solares. Química Nova, 30(1), 153–158. https://doi.org/10.1590/S0100-40422007000100027
6. Fernandez, C., Marti-Mestres, G., Ramos, J., & Maillols, H. (2000) LC analysis of benzophenone-3: II application to determination of “in vitro” and “in vivo” skin penetration from solvents, coarse and submicron emulsions. Journal of Pharmaceutical and Biomedical Analysis, 24(1), 155–165. https://doi.org/10.1016/S0731-7085(00)00399-X
7. Bolzinger, M.A., Briançon, S., Pelletier, J., & Chevalier, Y. (2012) Penetration of drugs through skin, a complex rate-controlling membrane. Current opinion in colloid & interface science, 17(3), 156–165. https://doi.org/10.1016/j.cocis.2012.02.001
8. Kasichayanula, S., House, J.D., Wang, T., & Gu, X. (2007) Percutaneous characterization of the insect repellent DEET and the sunscreen oxybenzone from topical skin application. Toxicology and Applied Pharmacology, 223(2), 187–194. https://doi.org/10.1016/j.taap.2007.05.016
9. Balaguer, A., Salvador, A., Chisvert, A., Meliá, M., Herráez, M., & Díez, O. (2006) A liquid chromatography-fluorimetric method for the in vitro estimation of the skin penetration of disodium phenyldibenzimidazole tetrasulfonate from sunscreen formulations through human skin. Analytical and Bioanalytical Chemistry, 385(7), 1225–1232. https://doi.org/10.1007/s00216-006-0344-2
10. Monteiro, M.S.S.B., Ozzetti, R.A., Vergnanini, A.L., Brito-Gitirana, L., Volpato, N.M., Freitas, Z.M.F., Ricci-Júnior, E., & Santos, E.P. (2012) Evaluation of octyl p-methoxycinnamate included in liposomes and cyclodextrins in anti-solar preparations: preparations, characterizations and in vitro penetration studies. International Journal of Nanomedicine, 7, 3045–3058. https://doi.org/10.2147/IJN.S28550
11. Souza, C., & Maia Campos, P.M.B.G. (2017) Development of a HPLC method for determination of four UV filters in sunscreen and its application to skin penetration studies. Biomedical Chromatography, 31(12), e4029. https://doi.org/10.1002/bmc.4029
12. Alvarez-Román, R., Naik, A., Kalia, Y., Guy, R., & Fessi, H. (2004) Enhancement of Topical Delivery from Biodegradable Nanoparticles. Pharmaceutical Research, 21(10), 1818–1825. https://doi.org/10.1023/b:pham.0000045235.86197.ef.
13. Bhuptani, R.S., & Patravale, V.B. (2019) Starch microsponges for enhanced retention and efficacy of topical sunscreen. Materials Science and Engineering C, 104, 109882. https://doi.org/10.1016/j.msec.2019.109882
14. Wissing, S.A., & Müller, R.H. (2002) Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. Journal of Controlled Release, 81(3), 225–233. https://doi.org/10.1016/S0168-3659(02)00056-1
15. Berbicz, F., Nogueira, A.C., Neto, A.M., Natali, M.R.M., Baesso, M.L., & Matioli, G. (2011) Use of photoacoustic spectroscopy in the characterization of inclusion complexes of benzophenone-3-hydroxypropyl-β-cyclodextrin and ex vivo evaluation of the percutaneous penetration of sunscreen. European Journal of Pharmaceutics and Biopharmaceutics, 79(2), 449–457. https://doi.org/10.1016/j.ejpb.2011.03.026
16. Adlhart, C., & Baschong, W. (2011) Surface distribution and depths profiling of particulate organic UV absorbers by Raman imaging and tape stripping. International Journal of Cosmetic Science, 33(6), 527–534. https://doi.org/10.1111/j.1468-2494.2011.00666.x
17. Bolzinger, M.A., Briançon, S., & Chevalier, Y. (2011) Nanoparticles through the skin: managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 3(5), 463–478. https://doi.org/10.1002/wnan.146
18. Brand, R., McMahon, L., Jendrzejewski, J., & Charron, A. (2007) Transdermal absorption of the herbicide 2,4-dichlorophenoxyacetic acid is enhanced by both ethanol consumption and sunscreen application. Food and Chemical Toxicology, 45(1), 93–97. https://doi.org/10.1016/j.fct.2006.08.005
19. Jiménez, M.M., Pelletier, J., Bobin, M.F., & Martini, M.C. (2004) Influence of encapsulation on the in vitro percutaneous absorption of octyl methoxycinnamate. International Journal of Pharmaceutics, 272(1–2), 45–55. https://doi.org/10.1016/j.ijpharm.2003.11.029
20. Puglia, C., Damiani, E., Offerta, A., Rizza, L., Tirendi, G.G., Tarico, M.S., Curreri, S., Bonina, F., & Perrotta, R.E. (2014) Evaluation of nanostructured lipid carriers (NLC) and nanoemulsions as carriers for UV-filters: Characterization, in vitro penetration and photostability studies. European Journal of Pharmaceutical Sciences, 51(1), 211–217. https://doi.org/10.1016/j.ejps.2013.09.023
21. Lademann, J., Jacobi, U., Surber, C., Weigmann, H.J., & Fluhr, J.W. (2009) The tape stripping procedure - evaluation of some critical parameters. European Journal of Pharmaceutics and Biopharmaceutics, 72(2), 317–323. https://doi.org/10.1016/j.ejpb.2008.08.008
22. Weigmann, H.J., Jacobi, U., Antoniou, C., Tsikrikas, G.N., Wendel, V., Rapp, C., Gers-Barlag, H., Sterry, W., & Lademann, J. (2005) Determination of penetration profiles of topically applied substances by means of tape stripping and optical spectroscopy: UV filter substance in sunscreens. Journal of Biomedical Optics, 10(1), 014009. https://doi.org/10.1117/1.1854683
23. Scalia, S., Battaglioli, S., & Bianchi, A. (2019) In vivo Human Skin Penetration of the UV Filter Ethylhexyl Triazone: Effect of Lipid Microparticle Encapsulation. Skin Pharmacology and Physiology, 32(1), 22–31. https://doi.org/10.1159/000493761
24. Potard, G., Laugel, C., Schaefer, H., & Marty, J.P. (2000) The Stripping Technique: In vitro Absorption and Penetration of Five UV Filters on Excised Fresh Human Skin. Skin Pharmacology and Physiology, 13(6), 336–344. https://doi.org/10.1159/000029941
25. Padula, C., Campana, N., & Santi, P. (2008) Simultaneous determination of benzophenone-3, retinol and retinyl acetate in pig ear skin layers by high-performance liquid chromatography. Biomedical Chromatography, 22, 1060–1065. https://doi.org/10.1002/bmc.1024
26. Fernandez, C., Marti-Mestres, G., Mestres, J.P., & Maillols, H. (2000) LC analysis of benzophenone-3 in pigskin and in saline solution: Application to determination of in vitro skin penetration. Journal of Pharmaceutical and Biomedical Analysis, 22(2), 393–402. https://doi.org/10.1016/S0731-7085(99)00277-0
27. Fernandez, C., Nielloud, F., Fortuné, R., Vian, L., & Marti-Mestres, G. (2002) Benzophenone-3: rapid prediction and evaluation using non-invasive methods of in vivo human penetration. Journal of Pharmaceutical and Biomedical Analysis, 28(1), 57–63. https://doi.org/10.1016/S0731-7085(01)00630-6
28. Couteau, C., Perez Cullel, N., Connan, A.E., & Coiffard, L.J.M. (2001) Stripping method to quantify absorption of two sunscreens in human. International Journal of Pharmaceutics, 222(1), 153–157. https://doi.org/10.1016/S0378-5173(01)00674-3
29. Gebauer, V., Weigmann, H.J., Schanzer, S., Meinke, M.C., Vergou, T., Sterry, W., & Lademann, J. (2012) Influence of skin aging effects on the skin surface profile and the correlated distribution of topically applied sunscreens. Journal of Biophotonics, 5(3), 274–282. https://doi.org/10.1002/jbio.201100104chatelain
30. Haque, T., Crowther, J.M., Lane, M.E., & Moore, D.J. (2016) Chemical ultraviolet absorbers topically applied in a skin barrier mimetic formulation remain in the outer stratum corneum of porcine skin. International Journal of Pharmaceutics, 510(1), 250–254. https://doi.org/10.1016/j.ijpharm.2016.06.041
31. Durand, L., Habran, N., Henschel, V., & Amighi, K. (2009) In vitro evaluation of the cutaneous penetration of sprayable sunscreen emulsions with high concentrations of UV filters. International Journal of Cosmetic Science, 31(4), 279–292. https://doi.org/10.1111/j.1468-2494.2009.00498.x
32. Oliveira, C.A.M. (2009) Estudos de permeação através da pele (Dissertação de Mestrado, Departamento de Química, Universidade de Aveiro, Portugal). Available: https://ria.ua.pt/bitstream/10773/4552/1/231152.pdf [Accessed: 17-abr-2023]
33. Vettor, M., Bourgeois, S., Fessi, H., Pelletier, J., Perugini, P., Pavanetto, F., & Bolzinger, M.A. (2010) Skin absorption studies of octyl-methoxycinnamate loaded poly(D,L-lactide) nanoparticles: Estimation of the UV filter distribution and release behaviour in skin layers. Journal of Microencapsulation, 27(3), 253–262. https://doi.org/10.3109/10717540903097770
34. Lin, Y.C., Lin, C.F., Alalaiwe, A., Wang, P.W., Fang, Y.P., & Fang, J.Y. (2018) UV filter entrapment in mesoporous silica hydrogel for skin protection against UVA with minimization of percutaneous absorption. European Journal of Pharmaceutical Sciences, 122, 185–194. https://doi.org/10.1016/j.ejps.2018.07.013
35. Sarveiya, V., Templeton, J., & Benson, H. (2004) Inclusion Complexation of the Sunscreen 2-Hydroxy-4-Methoxy Benzophenone (Oxybenzone) with Hydroxypropyl-b-Cyclodextrin: Effect on Membrane Diffusion. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 49(3–4), 275–281. https://doi.org/10.1007/s10847-004-6098-6
36. Cross, S.E., Jiang, R., Benson, H.A.E., & Roberts, M.S. (2001) Can Increasing the Viscosity of Formulations be used to Reduce the Human Skin Penetration of the Sunscreen Oxybenzone? Journal of Investigative Dermatology, 117(1), 147–150. https://doi.org/10.1046/j.1523-1747.2001.01398.x
37. Vilela, F.M.P., Fonseca, Y.M., Vicentini, F.T.M.C., Fonseca, M.J.V., & Do Amaral, M.D.P.H. (2011) Determination of three ultraviolet filters in sunscreen formulations and from skin penetration studies by high-performance liquid chromatography. Quimica Nova, 34(5), 879–883. https://doi.org/10.1590/S0100-40422011000500026
38. Chatelain, E., Gabard, B., & Surber, C. (2003) Skin Penetration and Sun Protection Factor of Five UV Filters: Effect of the Vehicle. Skin Pharmacology and Applied Skin Physiology, 16(1), 28–35. https://doi.org/10.1159/000068291
39. Hanno, I., Anselmi, C., & Bouchemal, K. (2012) Polyamide Nanocapsules and Nano-emulsions Containing Parsol® MCX and Parsol® 1789: In Vitro Release, Ex Vivo Skin Penetration and Photo-Stability Studies. Pharmaceutical Research, 29(2), 559–573. https://doi.org/10.1007/s11095-011-0592-5
40. Prado, A.H., Borges, M.C., Eloy, J.O., Peccinini, R.G., & Chorilli, M. (2017) An ultra-high performance liquid chromatography method to determine the skin penetration of an octyl methoxycinnamate-loaded liquid crystalline system. Pharmazie, 72(10), 563–567. https://doi.org/10.1691/ph.2017.7037
41. Olvera-Martínez, B., Cázares-Delgadillo, J., Calderilla-Fajardo, S., Villalobos-García, R., Ganem-Quintanar, A., & Quintanar-Guerrero, D. (2005) Preparation of polymeric nanocapsules containing octyl methoxycinnamate by the emulsification-diffusion technique: Penetration across the stratum corneum. Journal of pharmaceutical sciences, 94(7), 1552–1559. https://doi.org/10.1002/jps.20352
42. Mestres, J.P., Duracher, L., Baux, C., Vian, L., & Marti-Mestres, G. (2010) Benzophenone-3 entrapped in solid lipid microspheres: Formulation and in vitro skin evaluation. International Journal of Pharmaceutics, 400(1–2), 1–7. https://doi.org/10.1016/j.ijpharm.2010.07.028
43. Martins, R., Siqueira, S., Fonseca, M., & Freitas, L. (2014) Skin penetration and photoprotection of topical formulations containing benzophenone-3 solid lipid microparticles prepared by the solvent-free spray-congealing technique. Journal of Microencapsulation, 31(7), 644–653. https://doi.org/10.3109/02652048.2014.911378
44. Marcato, P., Caverzan, J., Rossi-Bergmann, B., Pinto, E., Machado, D., Silva, R., Justo, G., Ferreira, C., & Durán, N. (2011) Nanostructured Polymer and Lipid Carriers for Sunscreen. Biological Effects and Skin Permeation. Journal of Nanoscience and Nanotechnology, 11(3), 1880–1886. https://doi.org/10.1166/jnn.2011.3135
45. Siqueira, N.M., Contri, R.V., Paese, K., Beck, R.C.R., Pohlmann, A.R., & Guterres, S.S. (2011) Innovative Sunscreen Formulation Based on Benzophenone-3-Loaded Chitosan-Coated Polymeric Nanocapsules. Skin Pharmacology and Physiology, 24(3), 166–174. https://doi.org/10.1159/000323273
46. Scalia, S., Mezzena, M., & Ramaccini, D. (2011) Encapsulation of the UV Filters Ethylhexyl Methoxycinnamate and Butyl Methoxydibenzoylmethane in Lipid Microparticles: Effect on in vivo Human Skin Permeation. Skin Pharmacology and Physiology, 24(4), 182–189. https://doi.org/10.1159/000324054
47. Scalia, S., Coppi, G., & Iannuccelli, V. (2011) Microencapsulation of a cyclodextrin complex of the UV filter, butyl methoxydibenzoylmethane: In vivo skin penetration studies. Journal of Pharmaceutical and Biomedical Analysis, 54(2), 345–350. https://doi.org/10.1016/j.jpba.2010.09.018
48. Scalia, S., & Mezzena, M. (2009) Incorporation in Lipid Microparticles of the UVA Filter, Butyl Methoxydibenzoylmethane Combined with the UVB Filter, Octocrylene: Effect on Photostability. American Association of Pharmaceutical Scientists, 10(2), 384–390. https://doi.org/10.1208/s12249-009-9217-2
49. Calderilla-Fajardo, S.B., Cázares-Delgadillo, J., Villalobos-García, R., Quintanar-Guerrero, D., Ganem-Quintanar, A., & Robles, R. (2006) Influence of Sucrose Esters on the In Vivo Percutaneous Penetration of Octyl Methoxycinnamate Formulated in Nanocapsules, Nanoemulsion, and Emulsion. Drug Development and Industrial Pharmacy, 32(1), 107–113. https://doi.org/10.1080/03639040500388540
50. Zhou, Y., Qian, Y., Wang, J., Qiu, X., & Zeng, H. (2020) Bioinspired Lignin-Polydopamine Nanocapsules with Strong Bioadhesion for Long-Acting and High-Performance Natural Sunscreens. Biomacromolecules, 21(8), 3231–3241. https://doi.org/10.1021/acs.biomac.0c00696
51. Godwin, D.A., Kim, N.H., & Felton, L.A. (2002) Influence of Transcutol® CG on the skin accumulation and transdermal permeation of ultraviolet absorbers. European Journal of Pharmaceutics and Biopharmaceutics, 53(1), 23–27. https://doi.org/10.1016/S0939-6411(01)00215-6
52. Brand, R., Spalding, M., & Mueller, C. (2002) Sunscreens Can Increase Dermal Penetration of 2,4-Dichlorophenoxyacetic Acid. Journal of Toxicology. Clinical Toxicology, 40(7), 827–832. https://doi.org/10.1081/clt-120016952
53. Pont, A.R., Charron, A.R., & Brand, R.M. (2004) Active ingredients in sunscreens act as topical penetration enhancers for the herbicide 2,4-dichlorophenoxyacetic acid. Toxicology and Applied Pharmacology, 195(3), 348–354. https://doi.org/10.1016/j.taap.2003.09.021
54. Gu, X., Wang, T., Collins, D.M., Kasichayanula, S., & Burczynski, F.J. (2005) In vitro evaluation of concurrent use of commercially available insect repellent and sunscreen preparations. British Journal of Dermatology, 152(6), 1263–1267. https://doi.org/10.1111/j.1365-2133.2005.06691.x
55. Gu, X., Kasichayanula, S., Fediuk, D.J., & Burczynski, F.J. (2004) In-vitro permeation of the insect repellent N,N-diethyl-m-toluamide (DEET) and the sunscreen oxybenzone. Journal of Pharmacy and Pharmacology, 56(5), 621–628. https://doi.org/10.1211/0022357023402
56. Kasichayanula, S., House, J.D., Wang, T., & Gu, X. (2005) Simultaneous analysis of insect repellent DEET, sunscreen oxybenzone and five relevant metabolites by reversed-phase HPLC with UV detection: Application to an in vivo study in a piglet model. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, 822(1–2), 271–277. https://doi.org/10.1016/j.jchromb.2005.06.015
57. Freitas, J.V., Praça, F.S.G., Bentley, M.V.L.B., & Gaspar, L.R. (2015) Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. International Journal of Pharmaceutics, 484(1–2), 131–137. https://doi.org/10.1016/j.ijpharm.2015.02.062
58. Heo, S., Hwang, H.S., Jeong, Y., & Na, K. (2018) Skin protection efficacy from UV irradiation and skin penetration property of polysaccharide-benzophenone conjugates as a sunscreen agent. Carbohydrate Polymers, 195, 534–541. https://doi.org/10.1016/j.carbpol.2018.05.010
59. Rosado, C., Tokunaga, V.K., Sauce, R., Oliveira, C.A., Sarruf, F.D., Parise-Filho, R., Maurício, E., Almeida, T.S., Velasco, M.V.R., & Baby, A.R. (2019) Another Reason for Using Caffeine in Dermocosmetics: Sunscreen Adjuvant. Frontiers in Physiology, 10(519), 1–8. https://doi.org/10.3389/fphys.2019.00519
60. Simeoni, S., Scalia, S., & Benson, H.A.E. (2004) Influence of cyclodextrins on in vitro human skin absorption of the sunscreen, butyl-methoxydibenzoylmethane. International Journal of Pharmaceutics, 280(1–2), 163–171. https://doi.org/10.1016/j.ijpharm.2004.05.021
61. Yang, J., Wiley, C.J., Godwin, D.A., & Felton, L.A. (2008) Influence of hydroxypropyl-beta-cyclodextrin on transdermal penetration and photostability of avobenzone. European Journal of Pharmaceutics and Biopharmaceutics, 69(2), 605–612. https://doi.org/10.1016/j.ejpb.2007.12.015
62. Chen, W.Y., Fang, C.L., Al-Suwayeh, S., Yang, H.H., Li, Y.C., & Fang, J.Y. (2013) Risk assessment of excess drug and sunscreen absorption via skin with ablative fractional laser resurfacing. Lasers in Medical Science, 28(5), 1363–1374. https://doi.org/10.1007/s10103-012-1257-2
63. Li, C.C., Lin, Y.T., Chen, Y.T., Sie, S.F., & Chen-Yang, Y.W. (2015) Improvement in UV protection retention capability and reduction in skin penetration of benzophenone-3 with mesoporous silica as drug carrier by encapsulation. Journal of Photochemistry and Photobiology. B, Biology, 148 [s.n.], 277–283. https://doi.org/10.1016/j.jphotobiol.2015.04.027
64. Andréo-Filho, N., Bim, A.V.K., Kaneko, T.M., Kitice, N.A., Haridass, I.N., Abd, E., Lopes, P.S., Thakur, S.S., Parekh, H.S., Roberts, M.S., Grice, J.E., Benson, H.A.E., & Leite-Silva, V.R. (2018) Development and Evaluation of Lipid Nanoparticles Containing Natural Botanical Oil for Sun Protection: Characterization and in vitro and in vivo Human Skin Permeation and Toxicity. Skin Pharmacology and Physiology, 31(1), 1–9. https://doi.org/10.1159/000481691
65. Mota, A.C.V., Freitas, Z.M.F., Ricci Jr., E., Dellamora-Ortiz, G.M., Santos-Oliveira, R., Ozzetti, R.A., Vergnanini, A.L., Ribeiro, V.L., Silva, R.S., & dos Santos, E.P. (2013) In vivo and in vitro evaluation of octyl methoxycinnamate liposomes. International Journal of Nanomedicine, 8(1), 4689. https://doi.org/10.2147/IJN.S51383
66. Cozzi, A.C., Perugini, P., & Gourion-Arsiquaud, S. (2018) Comparative behavior between sunscreens based on free or encapsulated UV filters in term of skin penetration, retention and photo-stability. European Journal of Pharmaceutical Sciences, 121, 309–318.
67. Daneluti, A.L.M., Neto, F.M., Ruscinc, N., Lopes, I., Velasco, M.V.R., Matos, J.R., Baby, A.R., & Kalia, Y.N. (2019) Using ordered mesoporous silica SBA-15 to limit cutaneous penetration and transdermal permeation of organic UV filters. International Journal of Pharmaceutics, 570, 118633. https://doi.org/10.1016/j.ijpharm.2019.118633
68. Hayden, C.G.J., Cross, S.E., Anderson, C., Saunders, N.A., & Roberts, M.S. (2005) Sunscreen Penetration of Human Skin and Related Keratinocyte Toxicity after Topical Application. Skin Pharmacology and Physiology, 18(4), 170–174. https://doi.org/10.1159/000085861
69. Hung, C.F., Chen, W.Y., Aljuffali, I.A., Shih, H.C., & Fang, J.Y. (2014) The risk of hydroquinone and sunscreen over-absorption via photodamaged skin is not greater in senescent skin as compared to young skin: Nude mouse as an animal model. International Journal of Pharmaceutics, 471(1–2), 135–145. https://doi.org/10.1016/j.ijpharm.2014.05.034
70. Krishnan, R., Pradhan, S., Timares, L., Katiyar, S.K., Elmets, C.A., & Nordlund, T.M. (2006) Fluorescence of Sunscreens Adsorbed to Dielectric Nanospheres: Parallels to Optical Behavior on HaCat Cells and Skin. Photochemistry and Photobiology, 82(6), 1557–1565. https://doi.org/10.1562/2006-02-08-RA-800
71. Roussel, L., Gilbert, E., Salmon, D., Serre, C., Gabard, B., Haftek, M., Maibach, H.I., & Pirot, F. (2015) Measurement, analysis and prediction of topical UV filter bioavailability. International Journal of Pharmaceutics, 478(2), 804–810. https://doi.org/10.1016/j.ijpharm.2014.12.026
72. Sarveiya, V., Risk, S., & Benson, H.A.E. (2004) Skin penetration and systemic absorption of sunscreens after topical application. Journal of the American Academy of Dermatology, 50(3), P75–P75. https://doi.org/10.1016/j.jaad.2003.10.626
73. Tippavajhala, V.K., Mendes, T.O., & Martin, A.A. (2018) In Vivo Human Skin Penetration Study of Sunscreens by Confocal Raman Spectroscopy. American Association of Pharmaceutical Scientists, 19(2), 753–760. https://doi.org/10.1208/s12249-017-0852-8
