![]()
Biopharmaceutical Sciences, Biomed Biopharm Res., 2021; 18(2):252-262
doi: 10.19277/bbr.18.2.262; [+] PDF version here; [+] Portuguese html here
Effect of cosmetic films with ascorbic acid and hyaluronic acid on transepidermal water loss
Isadora Marques Garros Brait 1, Mariane Massufero Vergilio 2, Laura Moretti Aiello 1, Gislaine Ricci Leonardi 1*
1School of Pharmaceutical Sciences-University of Campinas (UNICAMP), 200, Cândido Portinari St., "Cidade Universitária Zeferino Vaz", 13083-871-Campinas, SP, Brazil; 2Graduate Program in Internal Medicine, School of Medical Sciences - University of Campinas (UNICAMP), 126, Tessália Vieira de Camargo St., "Cidade Universitária Zeferino Vaz", 13083-887-Campinas, SP, Brazil.
*corresponding author / autor para correspondência:
Abstract
Cosmetic films are structures made of a polymer capable of forming a continuous matrix. Collagen and hyaluronic acid (HA) are components of the skin extracellular matrix and act to maintain its elasticity and resistance. Ascorbic acid (AA) acts in the formation of collagen fibers, contributing to the maintenance of their firmness and elasticity. This study aimed to evaluate the moisturizing properties of cosmetic films with AA or HA in their composition. It was also investigated whether the presence of caprylyl glycol would interfere with the TWEL of the formulations. The occlusive properties of the films were assessed based on the reduction of transepidermal water loss (TEWL) in 20 healthy participants using a Tewameter probe. At T30, 30 minutes after application, all formulations showed a reduction in TEWL compared to the control. In general, the formulations that included caprylyl glycol presented the best TEWL results after 30 min of application. However, after 60 minutes, the transepidermal water loss began to return to its basal levels. This study showed a significant difference in the TEWL value, suggesting that the topical application of the films can contribute to the improvement and maintenance of skin hydration.
Keywords: cosmetic films; collagen; hyaluronic acid; ascorbic acid; skin barrier
Received: 13/08/2021; Accepted: 24/10/2021
Introduction
The presence of water in the skin is essential for all the body metabolic activities, its mechanical properties such as elasticity and turgidity (1), and therefore considered essential to the skin (2). Skin dehydration, in addition to initiating inflammatory processes, can lead to scaling, cracking, tension, redness, bleeding, sagging, and a worsening in the overall appearance of the skin (2).
Healthy skin remains hydrated by regulating the production of intracellular lipids and the natural moisturizing factor (NMF), which work by promoting the maintenance of water in the epidermis and dermis. However, factors such as water intake, hormone levels, metabolism, aging, and stress can affect hydration (1,2,3).
Moisturizing cosmetics can promote skin hydration, increasing the aqueous content of the stratum corneum or promoting the maintenance of water in this layer (2,3,4). This occurs due to humectant components, hygroscopic molecules that operate by attracting water to the upper layers of the skin, emollients, which fill the empty spaces between corneocytes, promoting a soft sensation, as well as occlusive agents, which prevent transepidermal water loss (TEWL) (3,4).
Several types of cosmetic formulations are developed to prevent and reverse skin dryness. Among them, cosmetic films are an innovative topical system, consisting of a polymer capable of forming a continuous matrix with a compact format, which allows the storage and transport of assets, with fewer inconveniences, in addition to being a biodegradable option (5). Currently, this system has also been widely used by the pharmaceutical industry in the production of transdermal drug delivery systems, dressings, and molds for tissue growth for the treatment of burns (6), by the industry of foods in the preservation of their products (7), and by the cosmetic industry to improve the performance of developed products.
Collagen is a structural biopolymer responsible for the constitution of skin, bones, blood vessels, extracellular matrix, teeth, and cartilage (8). In the skin, it is responsible for elasticity and resistance. As one of the main constituents of the extracellular matrix, its loss or reduction results in aging is signaled by the appearance of wrinkles and expression lines (9,10). Molecules or fibers of collegen protein can form hydrogels, films, or sponges that can be used as hemostatic agents, dressings, grafts, and molds for surgery and tissue growth (6).
Like collagen, hyaluronic acid (HA) is a component of the skin’s extracellular matrix (11), responsible for its firmness, turgor, and hydration, due to its ability to attract water molecules to this skin layer (10,12). During the aging process, levels of HA in the skin decrease (10,12). Its topical use is proven to be beneficial in healing processes, promoting faster and more uniform healing without causing deformities in the damaged skin (11,12,13,14).
Ascorbic acid (AA), or vitamin C, is an antioxidant and water-soluble molecule obtained through food or topical application (9). The latter has been shown to have more expressive effect in reducing spots on the skin than food supplementation, as observed in a study by Imai et al. (15). This vitamin also acts in the formation of collagen fibers in almost all structures, promoting the renewal of the skin barrier and thus contributing to the maintenance of its firmness and elasticity (9).
Caprylyl glycol is a diol that can act as an antimicrobial agent in cosmetic formulations due to its ability to destabilize the bacterial cell membrane. In the skin, it acts as a conditioning, emollient agent, filling the spaces between the corneocytes and making the skin softer, and as a humectant, attracting water molecules to the upper layers of the skin without generating hypersensitivity or irritation in humans (2,3,4,16,17).
To quantitatively assess the in vivo efficacy of cosmetic formulations and raw materials when applied to the skin, several important skin bioengineering techniques are used, including the measurement of the TEWL. TEWL is a natural process that occurs due to the gradient of water concentration between the layers of the skin, causing water to migrate from the deeper layers to the stratum corneum, where it is lost through evaporation (18). This property is one of the most important indicators of the integrity and functionality of the skin barrier, as the value of TEWL increases in damaged or destabilized skin (18).
Therefore, this study aimed to evaluate the moisturizing properties of cosmetic films composed of collagen, ascorbic acid, and hyaluronic acid by measuring TEWL with the aid of the skin bioengineering technique using a Tewameter® TW 210 probe (Courage-Khazaka electronic GmbH, Germany), coupled with the Multi Probe Adapter MPA 5 software.
Materials and Methods
Materials
In the development of cosmetic films, collagen (Gelita, Sao Paulo, BR), citric acid (Synth, Diadema, BR), ascorbic acid (Synth, Diadema, BR), hyaluronic acid (DSM, Sao Paulo, BR), caprylyl glycol (Fagron, São Paulo, BR), glycerin (Fagron, Sao Paulo, BR) and sodium metabisulfite (Synth, Diadema, BR) were used.
Development of cosmetic films
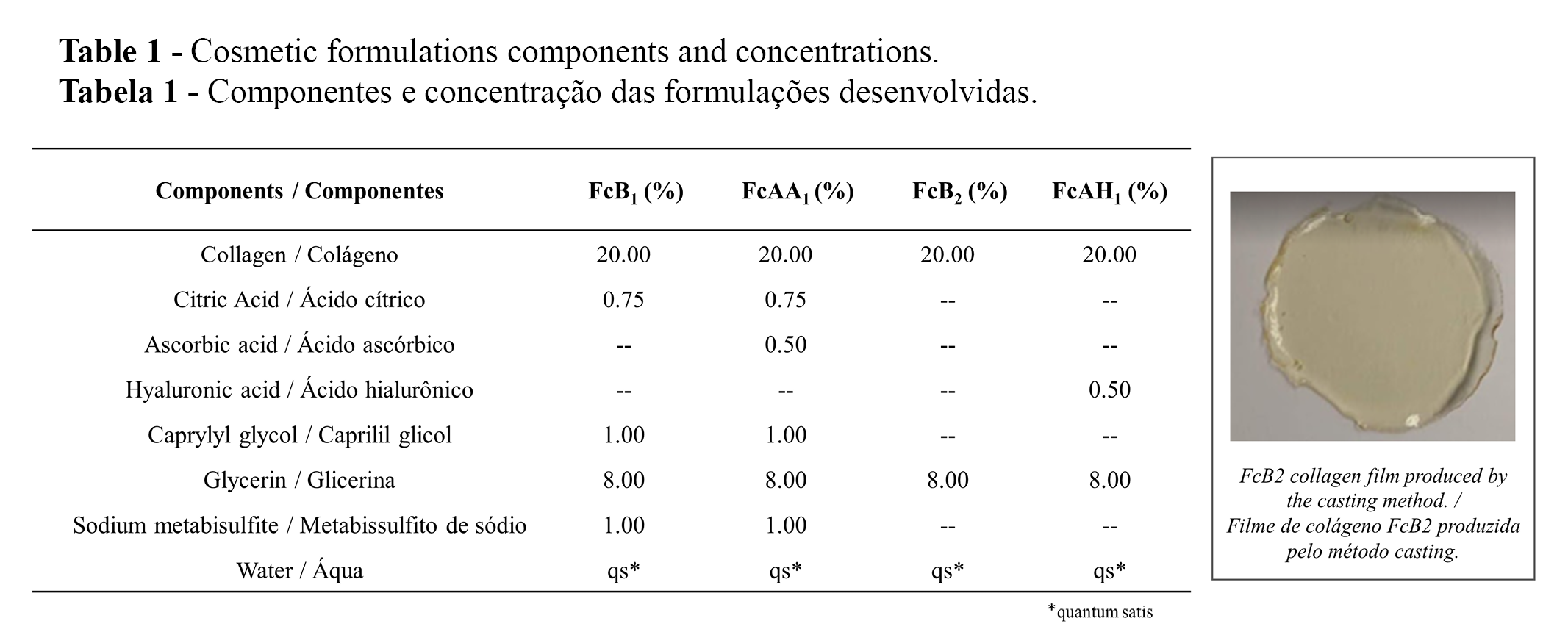
The preparation of four cosmetic formulations containing (or not) ascorbic acid and hyaluronic acid is summarized in Table 1. The formulations FcB1 and FcAA, without and with ascorbic acid, respectively, also contained caprylyl glycol. The cosmetic films (Table 1, inset) were prepared by casting method involving the addition of a filmogenic solution on a silicone surface followed by solvent evaporation at a constant temperature of 40 ºC (19).
In vivo efficacy assessment
Experimental design
This study was approved by the Research Ethics Committee of the School of Medical Sciences at Unicamp (CAAE: 13367219.5.0000.5404), and all participants signed forms to confirm informed consent. A total of 20 participants were included in the study according to the inclusion criteria, specifically, healthy participants aged between 20 and 60 years with the ability to understand and follow the guidelines, including not washing or applying any cosmetic products to the test region of the skin for at least two hours prior to testing. Exclusion criteria included the lack of understanding of the method to be used or presence of lesions compatible with active infectious diseases. The study was conducted in double-blind manner in a temperature-controlled room. The subjects were acclimated 20 minutes before starting instrumental measurements (20).
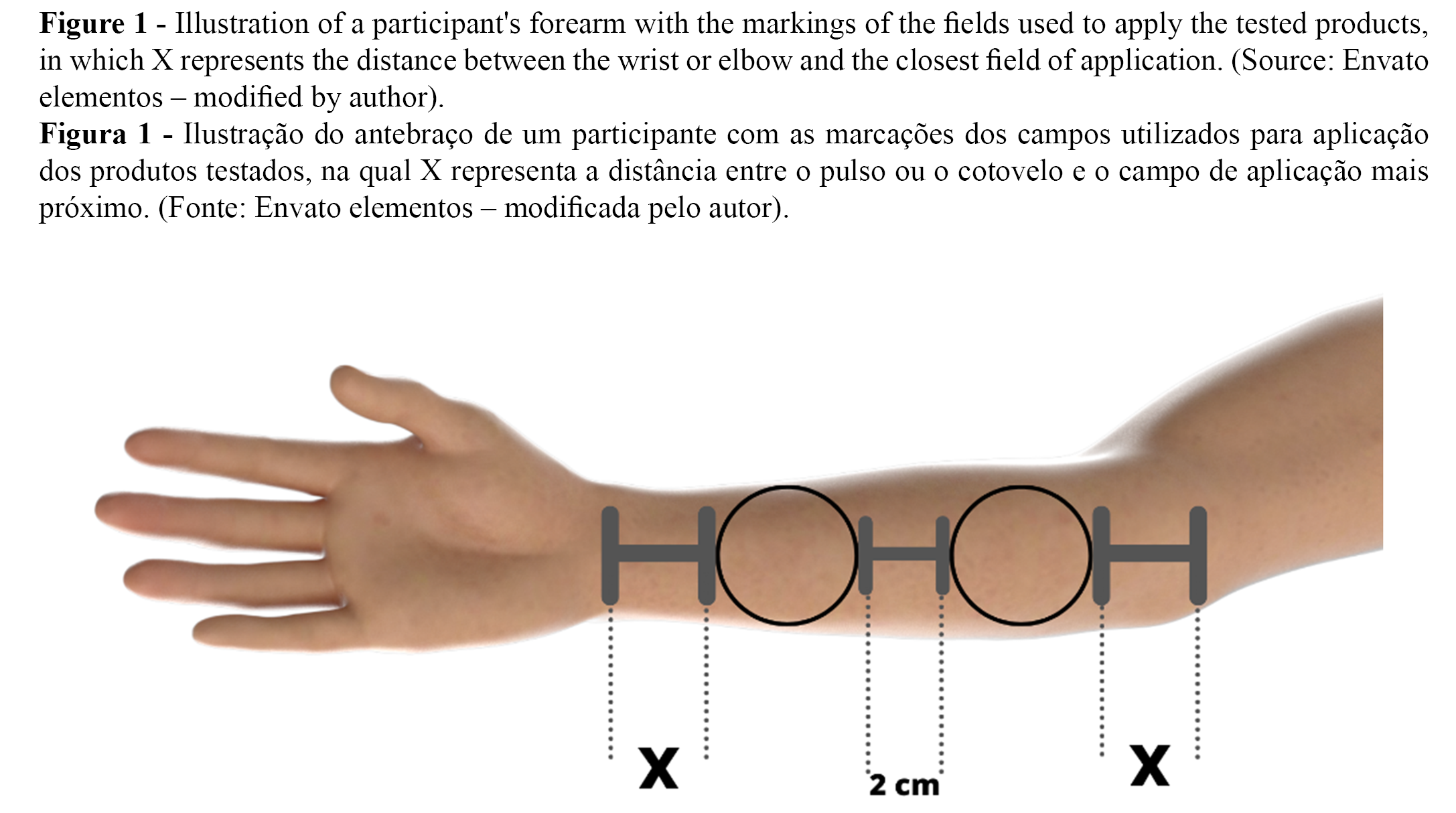
Five randomized sites with 16 cm² of area were marked on the volar forearms of each participant. The distance between the sites was 2 cm, as illustrated in Figure 1. As the formulations must be moistened to dissolve and release the active ingredients (5), the marked areas were moistened with ten drops of water before applying the films. The films were then randomly applied to the moistened regions by an independent researcher and removed after 15 minutes. Formulation residues were moistened with ten drops of water and spread on the skin for 30 seconds to ensure a more effective and uniform product absorption. Instrumental measurements were performed before (T0) and 30 min (T30), 60 min (T60) after application of the films, according to the EEMCO Guidance for the Assessment of TEWL in cosmetics (21).
Transepidermal water loss
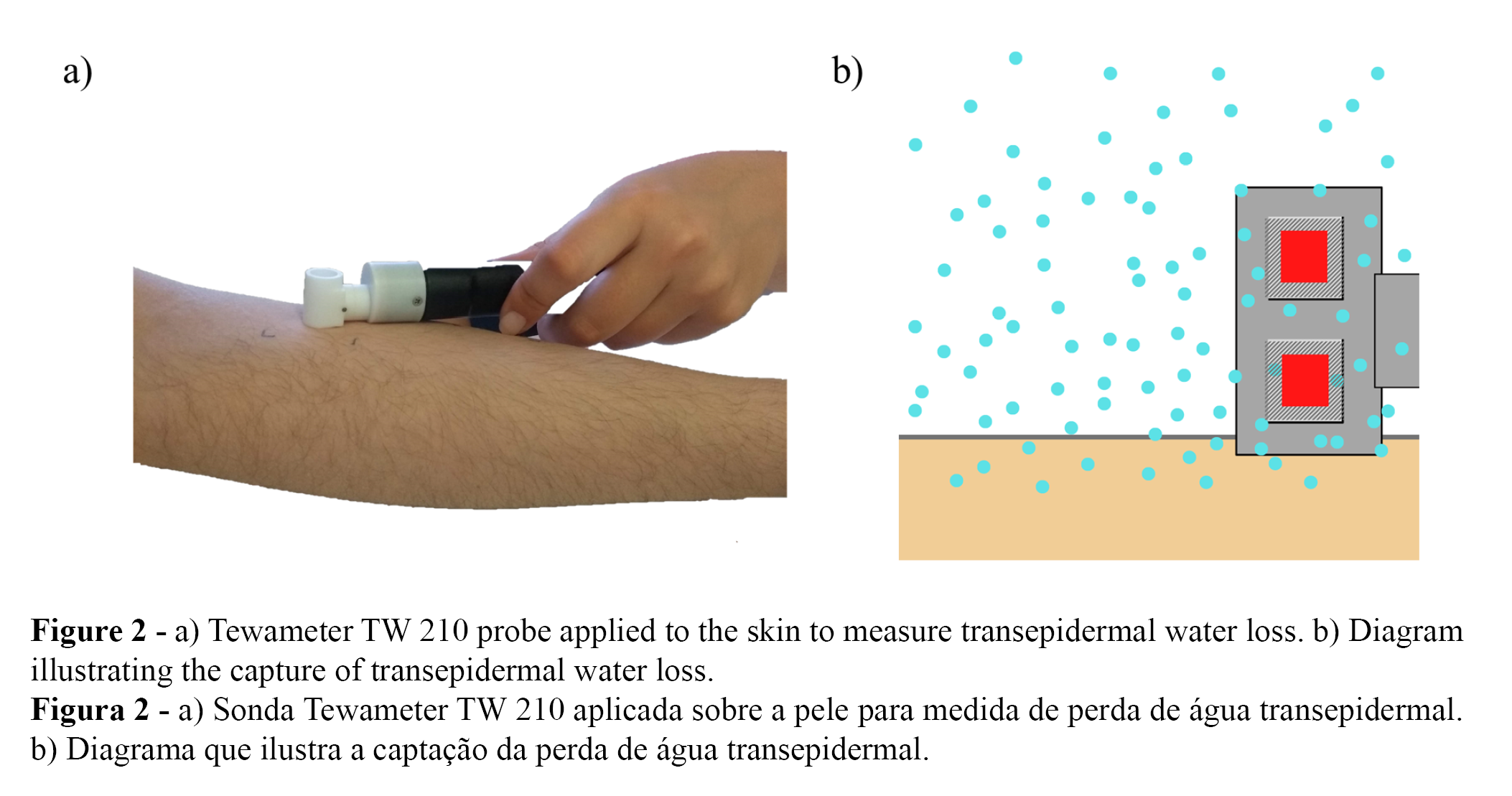
Skin barrier effect was evaluated through Transepidermal Water Loss (TEWL). TEWL measurements were performed as illustrated in Figure 2, using Tewameter TW 210 probe (Courage & Khazaka, Köln, Germany), whose function is to measure the transepidermal water loss based on the diffusion principle described by Adolf Fick in 1885, adapted by Capitani et al. (22):
𝑑𝑚/𝑑𝑡 = −𝐷×𝐴 × 𝑑𝑝/𝑑𝑥
Where dm/dt is the diffusion flux, A is the area, dc/dx is the concentration variation per distance, and D is the diffusion coefficient of water vapor in air. TEWL units are stated as g.m-2.h-1. The device probe was placed on the center of outlined skin areas and maintained in position to obtain 25 measurements (Figure 2).
Statistical analysis
One-way analyses of variance (ANOVA) were applied to test the differences among the formulations (α = 0.05). Tukey’s multiple comparison post-test was used to determine the differences among the obtained parameters. A confidence level of 95% was considered. Analyses were performed in GraphPad Prism 9.2.0 software (GraphPad, San Diego, LA, USA).
In order to compare the effects of different formulations on transepidermal water loss, the differences in the TEWL between the areas in which the films were applied and the control areas were calculated (23):
[(Tn – T0) – (NTn – NT0)]
Where Tn in the TEWL at time n, T30 or T60, T0 is the measure of TEWL at time T0, before application of the formulations, NTn is the measure of TEWL of the control area at time n and, NT0 is the measure of TEWL of the control area at time T0 (23). The differences were then divided by the value of Transepidermal Water Loss in T0 to obtain the increment percentage.
Results
The graphs shown in Figures 3 and 4 describe the averages of TEWL and the percentage difference of TEWL, respectively, in the evaluation of the cosmetic films at times T0, before application, and at 30 minutes (T30) and 60 minutes (T60) after application of the different films.
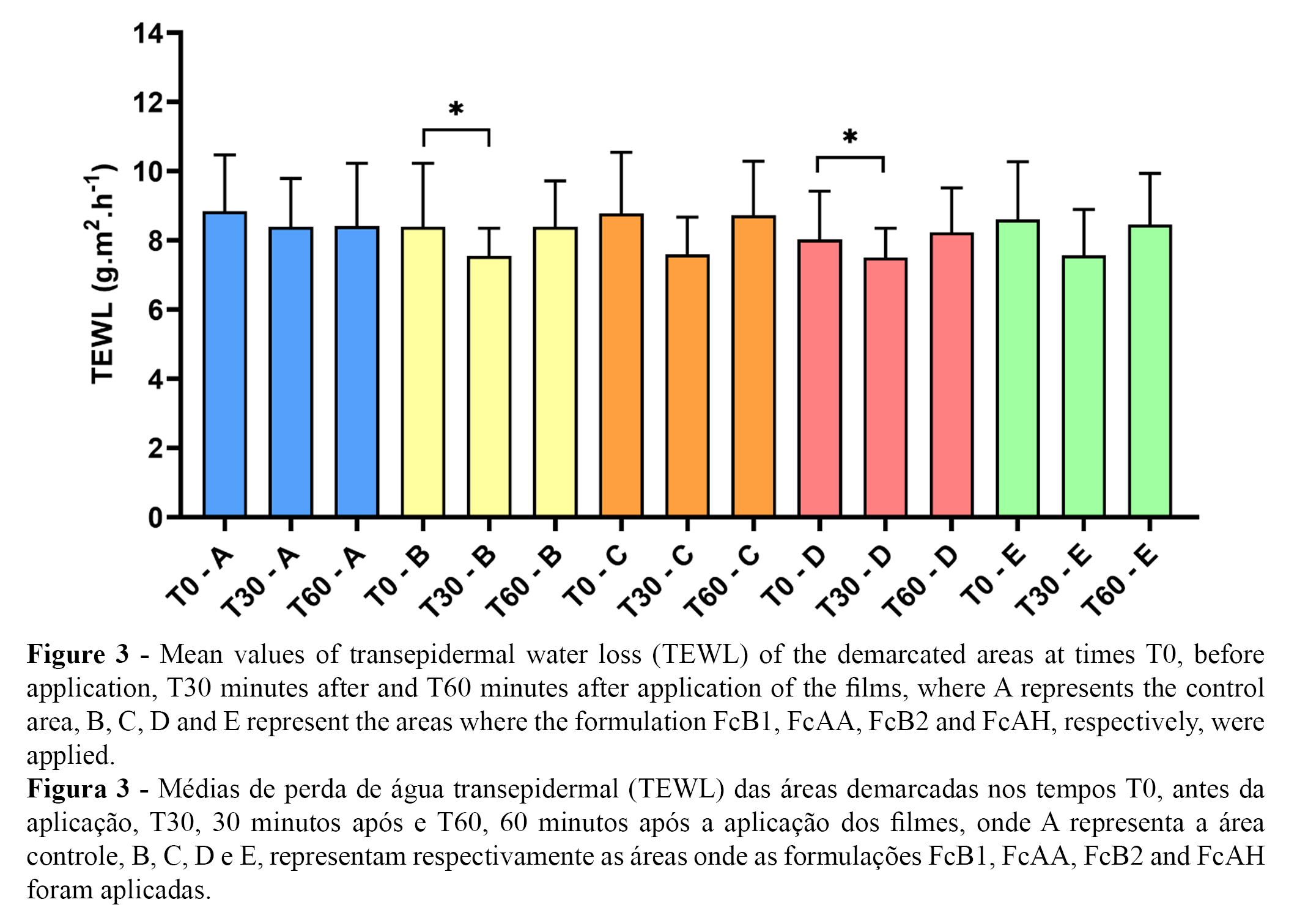
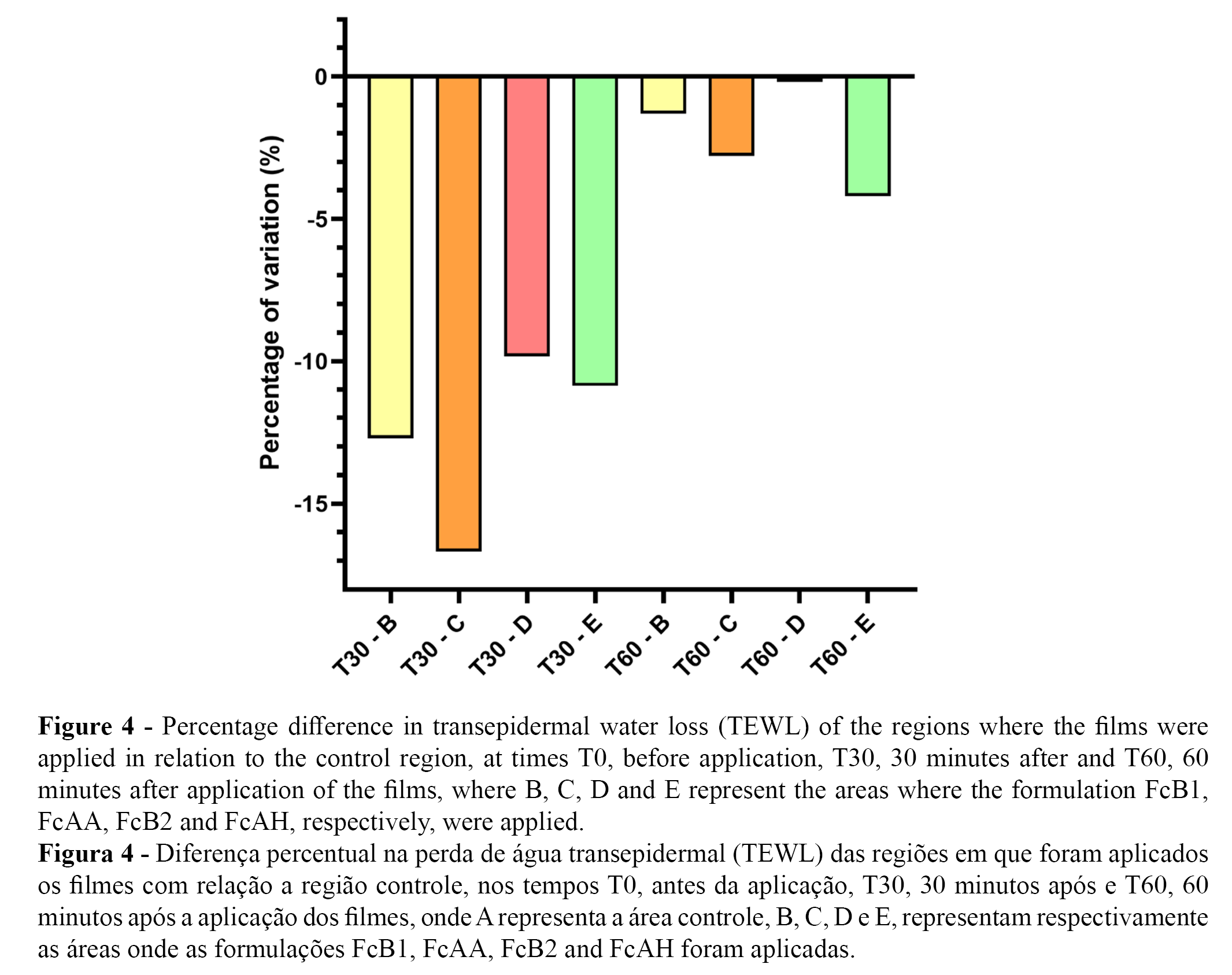
All formulations showed a reduction of at least -11% in the range of 0 to 30 minutes compared to the control. After 30 minutes of application, reduced the highest reduction in TEWL was recorded in the FcAA formulation, showing a -23.25 percent difference, followed by the FcB1 formulation, which reduced TEWL by 20.00%. TEWL was reduced 12.5% and 11% in the FcAH and FcB2 formulations, respectively.
In the interval between 0 to 60 minutes, the FcB1, FcAA, and FcAH formulations continued to show a reduction in TEWL compared to the control, with values of -5.00, -1.625 and -0.375%, respectively, while the FcB2 formulation showed an increase of 1.75%.
Discussion
The films developed in this work showed a barrier recovery effect demonstrated by the reduction in TEWL (3,4). Cosmetic films are capable to form a continuous matrix with a compact shape on the skin (5) and inhibit TEWL by occlusion; one of the hydration action mechanisms (24). The occlusive moisturizers decrease TEWL by forming a hydrophobic film on the skin surface and contributing to the corneocytes matrix (24).
The formulations including caprylyl glycol, FcB1 and FcAA, resulted in a more pronounced reduction in TEWL in both time intervals, showing better results as moisturizers than the formulations which did not include this ingredient. This TEWL decrease occurs because caprylyl glycol brings fatty material to the film-forming formulation, resulting in emollience and contributing to prevent transepidermal water loss on the skin surface (25). In the skin, the factor that most influences water loss is the composition and organization of lipids in the extracellular domains (26).
The decreased TEWL in the areas treated with formulations including caprylyl glycol or hyaluronic acid can also be explained by their humectant activity (10, 12). Those components attract water molecules to the skin surface (3, 4, 10, 12, 16, 17, 27), where they form an emulsion with skin lipids and sweat components, decreasing TEWL (27).
However, after 60 minutes, the TEWL values begin to return to basal levels, possibly due to product absorption. Furthermore, it is known that the reduction in TEWL values by the application of a topical formulation is related to the amount of product, the fatty content. and the lipid type present in the formulation (28). The addition of lipids with carbon chains of longer length provides lower skin barrier permeability and lower TEWL values (29). Thus, given that GC is a fatty alcohol with an intermediate carbon chain length, its emollient effect may have been reduced after a longer period of time (T60).
Further studies may explore the effects of cosmetic films added with emollients composed of longer fatty chains and other combinations of humectants in preserving and improving skin barrier function.
Conclusion
According to the observed results, it can be concluded that all the studied cosmetic films presented moisturizing power. Both the presence of caprylyl glycol and the presence of hyaluronic acid influenced the transepidermal water loss. For future studies, the importance of humectant and emollient components, as well as the size of the emollient molecules, must be taken into consideration.
Authors Contributions Statement
IMBG, conceptualization and study design; data analysis; drafting, editing and reviewing; MMV, conceptualization and study design; experimental implementation; drafting, editing and reviewing; LMA, data analysis; drafting, editing and reviewing; GRL, conceptualization and study design; supervision and final writing.
Funding
This study was financed in part by the National Council for Scientific and Technological Development (CNPq).
Acknowledgements
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 0001, and National Council for Scientific and Technological Development (CNPq).
Conflict of Interests
The authors declare there are no financial and/or personal relationships that could present a potential conflict of interests.
References
1. Mojumdar, E. H., Pham, Q. D., Topgaard, D., & Sparr, E. (2017). Skin hydration: interplay between molecular dynamics, structure and water uptake in the stratum corneum. Scientific reports, 7(1), 15712. https://doi.org/10.1038/s41598-017-15921-5
2. Dos Santos, A. K. S. et al. (2019). A importância da hidratação da pele na resposta ao tratamento da flacidez cutânea utilizando radiofrequência. Científica do Ciesa, 148.
3. Spada, F., Barnes, T. M., & Greive, K. A. (2018). Skin hydration is significantly increased by a cream formulated to mimic the skin’s own natural moisturizing systems. Clinical, Cosmetic and Investigational Dermatology, 11, 491–497. https://doi.org/10.2147/ccid.s177697
4. Downie, J. B. (2010). Understanding moisturizers and their clinical benefits. Practical Dermatology for Pediatrics, September/October, 19-22.
5. Truiti, M. D. C. T., & Sanfelice, A. M. (2010). Produtos em filme – Inovação na tecnologia de cosméticos. Acta Scientiarum. Health Science, 32(1). https://doi.org/10.4025/actascihealthsci.v32i1.6987
6. An, B., Lin, Y. S., & Brodsky, B. (2016). Collagen interactions: Drug design and delivery. Advanced Drug Delivery Reviews, 97, 69-84. https://doi.org/10.1016/j.addr.2015.11.013.
7. Lima, J.V., Moreira, M.N., Dos Santos, A.M., Brandão, M.R.S., De Aquino, A.A.(2020). Produção de Filme Ativo Adicionado de Óleo Essencial para Conservação de Queijos. Revista INGI-Indicação Geográfica e Inovação, 4 (2), 754-768.
8. Fratzl, P. (2008.). Collagen: Structure and Mechanics, an Introduction. In Collagen (pp. 1–13). Springer US. https://doi.org/10.1007/978-0-387-73906-9_1
9. Manela-Azulay, M., Mandrim-de-Lacerda, C.; Perez, M.A., Filgueira, A.L.& Cuzzi, T. (2003). Vitamina C / Vitamin C. Anais Brasileiros de Dermatologia, 3, 265.
10. Lee, D. H., Oh, I. Y., Koo, K. T., Suk, J. M., Jung, S. W., Park, J. O., Kim, B. J., & Choi, Y. M. (2014). Improvement in skin wrinkles using a preparation containing human growth factors and hyaluronic acid serum. Journal of Cosmetic and Laser Therapy, 17(1), 20–23. https://doi.org/10.3109/14764172.2014.968577
11. Belvedere, R., Bizzarro, V., Parente, L., Petrella, F., & Petrella, A. (2018). Effects of Prisma® Skin dermal regeneration device containing glycosaminoglycans on human keratinocytes and fibroblasts. Cell adhesion & migration, 12(2), 168–183. https://doi.org/10.1080/19336918.2017.1340137
12. Papakonstantinou, E., Roth, M., & Karakiulakis, G. (2012). Hyaluronic acid: A key molecule in skin aging. Dermato-Endocrinology, 4(3), 253–258. https://doi.org/10.4161/derm.21923
13. Rossi, S., Mori, M., Vigani, B., Bonferoni, M. C., Sandri, G., Riva, F., Caramella, C., & Ferrari, F. (2018). A novel dressing for the combined delivery of platelet lysate and vancomycin hydrochloride to chronic skin ulcers: Hyaluronic acid particles in alginate matrices. European Journal of Pharmaceutical Sciences, 118, 87–95. https://doi.org/10.1016/j.ejps.2018.03.024
14. Iacopetti, I., Perazzi, A., Martinello, T., Gemignani, F., & Patruno, M. (2020). Hyaluronic acid, Manuka honey and Acemannan gel: Wound-specific applications for skin lesions. Research in Veterinary Science, 129, 82–89. https://doi.org/10.1016/j.rvsc.2020.01.009
15. Imai, Y., Usui, T., Matsuzaki, T., Yokotani, H., & Mima, H. (1967). The antiscorbutic activity of L-ascorbic acid phosphate given orally and percutaneously in guinea pigs. Japanese journal of pharmacology, 17(2), 317–324. https://doi.org/10.1254/jjp.17.317
16. Lawan, K., Kanlayavattanakul, M., Lourith, N. (2009). Antimicrobial efficacy of caprylyl glycol and ethylhexylglycerine in emulsion. Journal of Health Research, 23 (1), 1-3.
17. Ziosi, P., Manfredini, S., Vandini, A. & Vertuani, S. (2013). Caprylyl glycol/phenethyl alcohol blend for alternative preservation of cosmetics. Cosmetics & Toiletries, 128, 538-551.
18. Jansen van Rensburg, S., Franken, A., & Du Plessis, J. L. (2019). Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Skin Research and Technology, 25(5), 595–605. https://doi.org/10.1111/srt.1271.
19. Roy, S., & Rhim, J.-W. (2020). Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocolloids, 98, 105302. https://doi.org/10.1016/j.foodhyd.2019.105302
20. Berardesca, E., & European Group for Efficacy Measurements on Cosmetics and Other Topical Products (EEMCO) (1997). EEMCO guidance for the assessment of stratum corneum hydration: electrical methods. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 3(2), 126–132. https://doi.org/10.1111/j.1600-0846.1997.tb00174.x
21. Rogiers, V. (2001) EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin research and technology : official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 14 (2), 117-128.
22. Capitani, R. D., Mercurio, D., Camargo Junior, F. B., & B. G. Maia Campos, P. M. (2012). Stability and Clinical Efficacy of Moisturizing Cosmetic Formulations Containing Vitamins C and E. Biomedical and Biopharmaceutical Research, 9(2), 215–224. https://doi.org/10.19277/bbr.9.2.44
23. Bazin, R., & Fanchon, C. (2006). Equivalence of face and volar forearm for the testing of moisturizing and firming effect of cosmetics in hydration and biomechanical studies. International journal of cosmetic science, 28(6), 453–460. https://doi.org/10.1111/j.1467-2494.2006.00352.x.
24. Kraft, J. N., & Lynde, C. W. (2005). Moisturizers: what they are and a practical approach to product selection. Skin therapy letter, 10(5), 1–8.
25. Correa, M. (2012) Cosmetologia: ciência e técnica. Medfarma, 67.
26. Lodén M. (2003). Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. American journal of clinical dermatology, 4(11), 771–788. https://doi.org/10.2165/00128071-200304110-00005
27. Gil-Castaño, G., & Cardona, R. (2020). Emolientes: beneficios, elementos clave y aplicación clínica. Revista alergia Mexico, 67, 128-141.. https://doi.org/10.29262/ram.v67i2.730.
28. Buraczewska, I., Broström, U., & Lodén, M. (2007). Artificial reduction in transepidermal water loss improves skin barrier function. British Journal of Dermatology, 157(1), 82–86. https://doi.org/10.1111/j.1365-2133.2007.07965.x
29. Petry, T., Bury, D., Fautz, R., Hauser, M., Huber, B., Markowetz, A., Mishra, S., Rettinger, K., Schuh, W., & Teichert, T. (2017). Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicology letters, 280, 70–78. https://doi.org/10.1016/j.toxlet.2017.07.899
