| Original Article, Biomed Biopharm Res., 2023; 20(1):64-82 doi: 10.19277/bbr.20.1.311; PDF version [+]; Portuguese html [PT] |
Methanol extract of Bauhinia forficata leaves reduced serum creatinine level and prevented the elevation of hepatic enzymes in mice exposed to gentamicin and acetaminophen: an exploratory study
Sebastián Funes-Rivera 1,2 ![]() , María L. Kennedy 1
, María L. Kennedy 1 ![]() , Antonia K. Galeano 1
, Antonia K. Galeano 1 ![]() , Patricia M. Funes Torres 2
, Patricia M. Funes Torres 2 ![]() & Miguel A. Campuzano-Bublitz 1
& Miguel A. Campuzano-Bublitz 1 ![]() ✉️
✉️
1 - Departamento de Farmacología, Facultad de Ciencias Químicas, Universidad Nacional de Asunción. Campus UNA, 2169. San Lorenzo. Paraguay
2 - Departamento de Bioquímica Clínica, Instituto de Investigaciones en Ciencias de la Salud, Universidad Nacional de Asunción. Campus UNA, 2169. San Lorenzo. Paraguay
Abstract
Kidney and liver diseases are a public health problem worldwide. Pharmacological management usually prevents its progress even if indirectly. Currently, more alternative treatments based on traditional medicine and medicinal plants are considered. Bauhinia forficata is widely distributed in South America and is used for its hepatoprotective and nephroprotective properties. This study was designed to preliminarily assess the nephroprotective and hepatoprotective effect of the methanol extract of B. forficata leaves (50, 100, 200, and 400 mg/kg, per os) in Swiss albino mice. Acute kidney injury was induced with gentamicin (135 mg/kg, intraperitoneal), followed by determination of creatinine, urea, uric acid, and electrolyte levels in urine and serum. Acute liver damage was induced by acetaminophen (300 mg/kg, intraperitoneal), followed by determination of a serum liver profile of aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. All tested doses of the extract were able to induce a significant reduction in serum creatinine levels, compared to untreated animals. Moreover, the extract effectively prevented the elevation of hepatic enzyme activity induced by acetaminophen. It is concluded that the extract of B. forficata leaves has nephroprotective and hepatoprotective effects in a mouse model of hepato and nephrotoxicity, which is compatible with traditional use.
Keywords: Bauhinia forficata, silymarin, creatinine, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase
To Cite: Funes-Rivera, S. F., Kennedy, M. L., Galeano, A. K., Funes Torres, P. M. & Campuzano-Bublitz, M. A. (2023) Methanol extract of Bauhinia forficata leaves reduced serum creatinine level and prevented the elevation of hepatic enzymes in mice exposed to gentamicin and acetaminophen: an exploratory study. Biomedical and Biopharmaceutical Research, 20(1), 64-82.
Correspondence to:
Received 17/04/2023; Accepted 22/06/2023
Introduction
The kidneys and liver are organs closely related to substance metabolism and detoxification. Stress situations, such as metabolic overload or frequent exposure to harmful agents, particularly the action of xenobiotics, compromise their proper function (1, 2). Few therapeutic resources are available, so the study of alternative medications to preserve the functionality of these organs is very important (3).
The etiology of kidney disease is variable; some are specific to the individual according to physiological factors; others are related to underlying pathologies, such as diabetes or high blood pressure, or are due to direct factors such as nephrotoxic compounds. Among these, the main compounds are aminoglycoside antibiotics (2). Kidney disease is among the first ten leading causes of death worldwide, regardless of race, sex, age, or geographical location. In our country, Paraguay, a total of 366 patients required hemodialysis in 2010. In 2019, the total number of dialyzed patients increased to 1638, of which 61% were men (4). Worldwide, 5 million people die annually from lack of access to critical treatments for kidney disease. It is projected that chronic kidney disease will soon be the fifth leading cause of death. Generally, with limited epidemiological data, limited knowledge, and poor access to laboratory services, the true burden of kidney disease is underestimated. Kidney diseases are pathologically diverse and are often asymptomatic. As such, it is often diagnosed late, and patient care requires specialized resources that increase healthcare costs. Prevention of kidney disease is highly cost-effective but requires a multisectoral holistic approach (5).
The liver is a critical organ in the human body, responsible for various functions, such as bile secretion. It is also related to the metabolism of carbohydrates, lipids, and proteins, the processing of drugs and hormones, and the detoxification of substances such as alcohol, drugs and hormones through a process called biotransformation (6). Liver disease is multifactorial, and may be the result of metabolic imbalances, such as alpha-1-antitrypsin deficiency, hemochromatosis (Wilson's disease), obesity, accumulation of drugs such as paracetamol or other xenobiotics in the body, or due to viruses. Thus, it is a disease that can occur in individuals of all ages. One of the main chronic liver diseases worldwide is cirrhosis, derived from obesity or long-term abuse of alcoholic beverages, which mainly affects individuals between 60 and 80 years of age (7). Liver disease accounts for over 2 million deaths annually (cirrhosis, viral hepatitis, and liver cancer), 4% of all deaths. Currently, liver disease is the 11th leading cause of death, but liver deaths may be underestimated (7).
Current pharmacological treatments mainly treat these conditions indirectly, reducing the factors that can lead to more significant complications, with certain side effects and high costs. Silymarin is a mixture of flavonolignans extracted from the seeds of Silybum marianum used since ancient times to treat chronic and acute liver conditions. It has been shown to prevent liver damage caused by various toxins, with its effects frequently attributed to its antioxidant, free radical scavenging, and iron chelating properties (8).
Several plants have shown their nephroprotective properties in experimental studies in animal models, among them Phyllanthus amarus (9), green tea (10), and Eclipta alba (11). Likewise, the aqueous extract of the bark of Bauhinia variegata demonstrated protective effects against liver failure and nephrotoxicity caused by thioacetamide in rats (12) and those with liver damage caused by carbon tetrachloride (13). Smallanthus sonchifolius reversed acetaminophen-induced liver damage in rats (14) and Opuntia ficus-indica cladodes extract prevented lithium-induced liver injury (15). In previous works, we have demonstrated the hepato- and nephroprotective property of Prosopis ruscifolia (16), Baccharis crispa (17), and Dorstenia brasiliensis (18) in mice.
Bauhinia forficata (Fabaceae) is an arboreal species whose leaves are used in infusion to treat kidney and liver conditions and as a hypoglycemic agent. In Brazil, it is used as a hypolipidemic, diuretic, expectorant, tonic, carminative, and digestive, among other uses (19). It is also used topically as an astringent to treat wounds or canker sores.
The methanol extract of B. forficata showed good antioxidant and hypoglycemic activities (19). Based on its terpenoid composition, its antibacterial and fungicidal activity has been verified. The traditional medicinal use of the infusion of B. forficata leaves has not reported cases of toxic adverse effects (19), although in vitro studies have shown that depending on the dose, mitochondrial damage, as well as mutagenic activities and thyroid dysfunction, can occur (20, 21).
The main constituents of B. forficate in leaves are phenolic compounds, particularly flavonoids, together with terpenes and steroids. The flavonoids are principally kaempferol and quercetin derivatives. Some phenolic compounds, including gallic acid, chlorogenic acid, and caffeic acid, were also identified (19, 22).
The use of natural therapies for several diseases has a long history, and B. forficate has been traditionally used to treat liver and kidney conditions. The current work aimed to evaluate the nephroprotective and hepatoprotective potential of Bauhinia forficata leaf extract. Nephroprotection was analyzed by determining classic renal profiles as well as urinary and renal electrolyte profiles. The hepatoprotective effect was evaluated via the hepatic profiling. Considering the importance of kidney and liver-associated health conditions, the potential protective effect in mice using methanol extract from this natural resource is reported here for the first time. The extract was able to attenuate chemically induced kidney and liver damage in mice evidenced by reduced serum creatinine levels and prevention of elevated hepatic enzyme activity.
Material and Methods
Plant material and extract preparation
The leaves of Bauhinia forficata Link (Fabaceae) were collected from the city of Itá, Central Department, Paraguay. The plant material was identified by researchers from the Department of Botany. A pressed and dried specimen is filed in the herbarium of the Facultad de Ciencias Químicas of Universidad Nacional de Asunción (FCQ-UNA), under the Code: Degen, González et González 4045. The extract of B. forficata was obtained by sonicating the dried and ground leaves in methanol (x3). The solvent was removed under reduced pressure on a rotary evaporator. The resulting extract was stored in a desiccator until used in biological assays. For this purpose, the extract was dissolved in water.
Reagents and equipment
Gentamicin (Larjan, Veinfar, Argentina), acetaminophen (Sigma, St. Louis, MO, USA)mosilymarin (Sigma, St. Louis, MO, USA) and sodium pentobarbital from Abbott (Japan) were used. Methanol was purchased locally and distilled before use. The estimations of parameters of hepatic and kidney functionality were made with equipment (Autoanalyzer CB350i) and kits purchased from Wiener Lab (Rome, Italy). Electrolyte determinations were made with equipment (Combi-line) and reagents from Eschweiler (Kiel, Germany). A Hermle Z216M microcentrifuge was used (HERMLE Labortechnik GmbH, Wehingen, Germany). Metabolic cages were purchased from Suzhou Fengshi Laboratory Animal Equipment Co. Ltd (Suzhou City, China). Normal and pathological human serum (Humatrol, Wiesbaden-Germany) were used as quality controls.
Experimental animals and ethical issues
Toxicity tests were performed with female mice. Nephro- and hepatoprotection experiments were performed in adult (12 weeks old) male Swiss albino mice weighing 30 ± 5 g, apparently healthy, procured from the animal facility of the Facultad de Ciencias Químicas, kept in standard laboratory conditions (acclimated to a temperature of 22 ± 2°C and humidity of up to 60%, in rooms with 12/12h light/darkness cycles). The animals were fed daily (6 g/animal) and received water ad libitum throughout the experiment. For animal handling, the standards established by the Ethics Commission of the European Community were followed (23). The project protocol was approved by the Research Ethics Committee of the FCQ (Comité de Ética en Investigación, CEI 471/19). The animals were used only once, and for their final disposal, they were delivered to a company specialized in biological waste management.
Acute oral toxicity test
Toxicity test guidelines in protocol number 420 of the Organization for Economic Cooperation and Development (OECD) were followed. A group of five female mice received orally, cumulatively, every 24 hours, doses of 5 up to 2000 mg/kg of extract and were compared with a control group (n=5, distilled water). The animals were observed up to 8 hours after each treatment and subsequently for a period of 15 days. The presence of signs of toxicity, as well as their severity, progression, and reversibility, were sought and recorded in relation to dose and time. At the end of the observation period, the main organs were macroscopically examined (24).
Gentamicin-induced nephrotoxicity and treatments
The doses for assessment of the nephroprotective and hepatoprotective effect in mice were selected according to the higher doses tested in acute toxicity tests. In this case, no evidence of toxicity was observed in doses lower than 2000 mg/kg. Male mice divided into seven groups (n=6) received the treatment for nine days: control (Veh; water, per os); gentamicin (Gent; water 0.1 mL/10g body weight, per os, and gentamicin); reference hepatoprotection drug silymarin (Sil; 150 mg/kg body weight, silymarin, dissolved in ethanol: propylene glycol: water 1:4:5, per os, and gentamicin); Bf 50, Bf 100, Bf 200, Bf 400 (containing 50, 100, 200 and 400 mg/kg of B. forficata methanol extract, respectively, per os, and gentamicin. Except in the control group, mice received the gentamicin (135 mg/kg; intraperitoneal) one hour after the treatments. This dose was standardized in previous studies in the mice procured from the animal facility of the Facultad de Ciencias Químicas. The weight of each mouse was recorded daily. On the ninth day, the mice were transferred to individual metabolic cages for 24 h to collect urine samples and quantify food consumption (16). The amount of food and the volume of water consumed during confinement in a metabolic box were also recorded. After the stay in metabolic cages, all animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and blood was collected.
Acetaminophen-induced hepatotoxicity and treatments
Mice were divided into seven groups (n=6). They were treated for four days: control (Veh; water, per os); acetaminophen (APAP; water, per os); silymarin (Sil; 150 mg/kg of silymarin, dissolved in ethanol: propylene glycol: water 1:4:5, per os); Bf 50, Bf 100, Bf 200, Bf 400 (with 50, 100, 200 and 400 mg/kg of B. forficata extract, respectively, per os). On the fourth day, two hours after the oral treatment, except animals in control group, acute hepatotoxicity was induced in mice using acetaminophen (APAP, 300 mg/kg, intraperitoneal). This dose was standardized in previous studies in the mice procured from the animal facility of the Facultad de Ciencias Químicas (16). Three hours after acetaminophen administration, mice were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and blood was collected. Alkaline phosphatase (ALP), aspartate aminotransferase (glutamic-oxaloacetic transaminase, GOT) and alanine aminotransferase (glutamic-pyruvic transaminase, GPT) were determined (16) in serum.
Determination of biochemical parameters
In the nephroprotective activity assay, exsanguination of mice by cardiac puncture under anesthesia was made following removal from the metabolic cages. Serum was obtained after incubation of the fresh blood for 20 min at 37°C and centrifugation for 10 minutes at 3000 rpm. The concentrations of urea, creatinine, and uric acid (mg/dL), as well as potassium and sodium (mEq/L) were determined. The volume of urine after 24 hours was measured (mL), and in this sample, the concentrations of creatinine, urea, and uric acid (mg/kg/24h), together with sodium and potassium (mEq/kg/24h) were determined. The concentration of sodium and potassium in serum and urine was determined in the Eschweiler Combi-Line electrolyte meter. The equipment was duly calibrated and accredited under internal and external quality controls prior to the determination of the analytes. The direct ion selective electrode method was used for serum determinations, referring to the determination of electrolytes in whole samples without dilution. For the determination of electrolytes in urine, the indirect ion selective electrode method was used, where the sample undergoes a dilution prior to the determination.
In the hepatoprotective activity assay, GOT, GPT, and ALP were determined. Serum was obtained after incubating the fresh blood and then centrifuged. Internal quality control made using normal and pathological control serum. The activities of GOT, GPT and ALP were determined in a spectrophotometer at 340nm, and the results expressed in U/L (25, 26).
Statistical analysis
The results correspond to mean ± standard deviation (SD), p<0.05 was considered statistically significant. One way ANOVA, and Dunnett`s test was done using the GraphPad Prism 7.0. (GraphPad Software, Inc., CA).
Results
Toxicity test results indicated that the methanol extract of Bauhinia forficata did not show a toxic effect during the observation period. No mortality was observed. No macroscopic differences or evidence of damage were observed between the organs (liver, lung, pancreas, heart, kidney) of animals in the control group and those treated with extract. The behavior of the animals was not altered. Moreover, water and food consumption in the treated and control groups were similar. Taken together these results indicate that the extract is safe up to 2000 mg/kg after oral administration (data not shown).
Determination of the nephroprotective effect
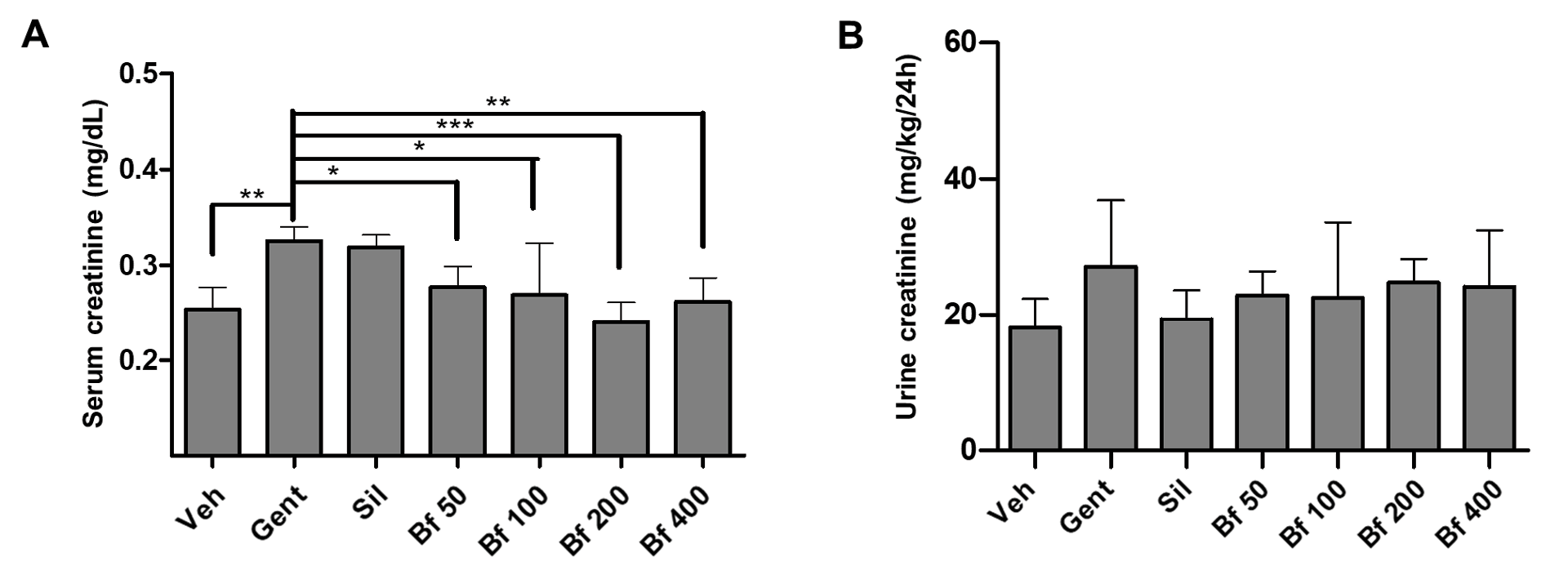
Male mice with kidney damage induced by gentamicin were used. The main marker of acute kidney injury is elevated serum creatinine concentration. After nine days, a statistically significant difference (Gent group 40% more than Veh, < 0.01) was observed between control (Veh) and pathological groups (Gent), validating this model to identify plant extracts with nephroprotective activity. A mild protective effect was observed in the group treated with silymarin, since serum creatinine level in this group was lower than in pathological group, but this difference was not significant. All tested doses of the extract significantly reduced the serum creatinine level (Bf 50 (78%) and Bf 100 (77%): p<0.05; Bf 200 (63%): p< 0.001; Bf 400 (71%): p<0.01; Figure 1 A), compared to Gent group. Urinary creatinine levels were not different, although the level in all groups treated with silymarin and the extract, were comparable to the value in control group and lower than the pathological group (Figure 1 B).
| Figure 1 - Effect of Bauhinia forficata on the concentration of creatinine in serum (A) and urine (B), in mice treated with Veh: water, Gent: gentamicin, Sil: silymarin, Bf 50, Bf 100, Bf 200 and Bf 400 (B. forficata 50, 100, 200 and 400 mg/kg, p.o.). Data are expressed as mean ± SD (n=6), one-way ANOVA, post hoc Dunnett's test. * p<0.05, ** p<0.01, ***p<0.001 significantly different from Gent). |
 |
Serum and urinary urea levels (Figures 2 A and B) were not altered after the treatment with B. forficata, when compared with the Gent group. Although serum urea levels showed a reduction in animals treated with all doses of the extract, this difference was not significant (Figure 2 A).
| Figure 2 - Effect of Bauhinia forficata on the concentration of urea in serum (A) and urine (B), in mice treated with Veh: water, Gent: gentamicin, Sil: silymarin, Bf 50, Bf 100, Bf 200 and Bf 400 (B. forficata 50, 100, 200 and 400 mg/kg, p.o.). Data are expressed as mean ± SD (n=6), one-way ANOVA, post hoc Dunnett's test. * p<0.05 significantly different from Gent. |
 |
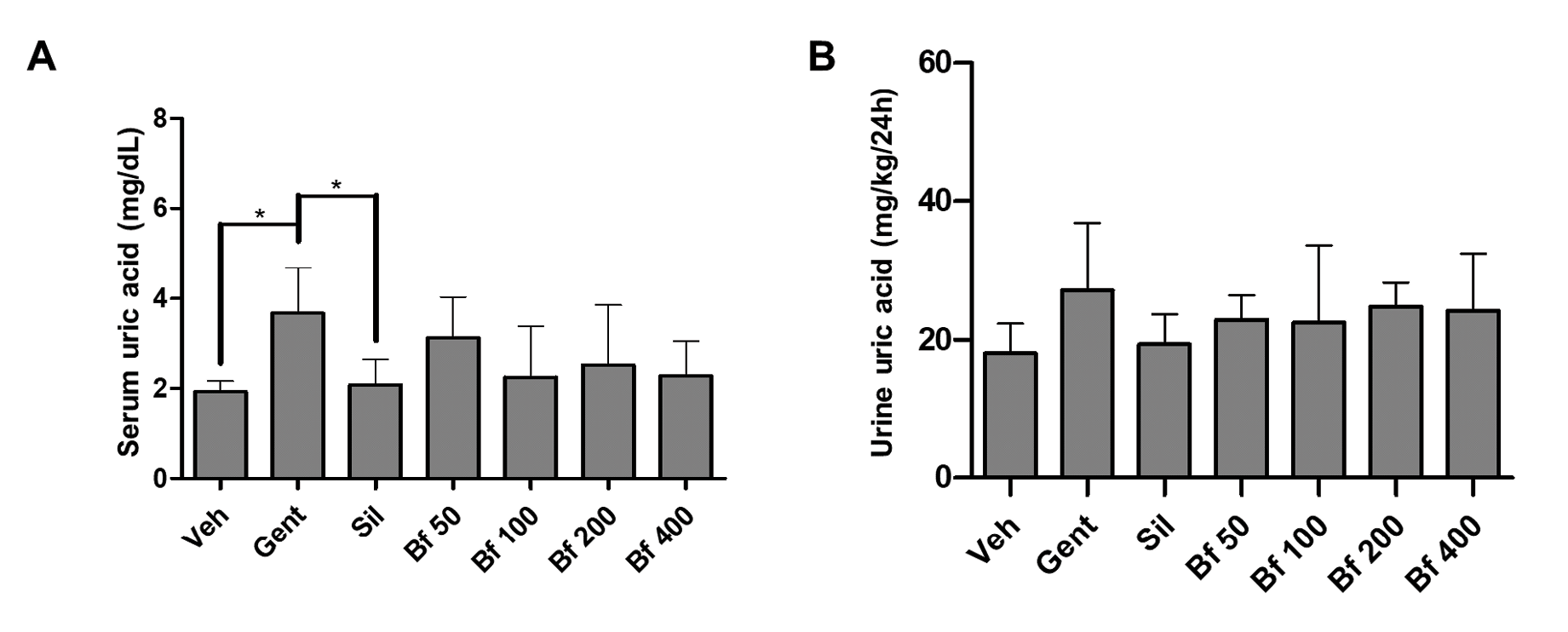
Pathological group (Gent) serum uric acid concentration was significantly higher compared to the control (86%, p<0.05), and silymarin (64%, p<0.05). Animals treated with the extract (50, 100, 200 and 400 mg/kg), exhibited lower concentrations than the Gent group and were closer to the silymarin (Sil) group. The levels of uric acid in urine were similar in all groups (Figures 3A and 3B). No differences were seen in serum and urine sodium and potassium levels (Figures 4 A-D).
| Figure 3 - Effect of Bauhinia forficata on the concentration of uric acid in serum (A) and urine (B), in mice treated with Veh: water, Gent: gentamicin, Sil: silymarin, Bf 50, Bf 100, Bf 200 and Bf 400 (B. forficata 50, 100, 200 and 400 mg/kg, p.o.). Data are expressed as mean ± SD (n=6), one-way ANOVA, post hoc Dunnett's test. * p<0.05 significantly different from Gent. |
 |
| Figure 4 - Effect of Bauhinia forficata on the concentration of sodium in serum (A) and urine (B), and of poassium in serum (C) and urine (D), in mice treated with Veh: water, Gent: gentamicin, Sil: silymarin, Bf 50, Bf 100, Bf 200 and Bf 400 (B. forficata 50, 100, 200 and 400 mg/kg, p.o.). Data are expressed as mean ± SD (n=6), one-way ANOVA, post hoc Dunnett's test. * p<0.05 significantly different from Gent. |
 |
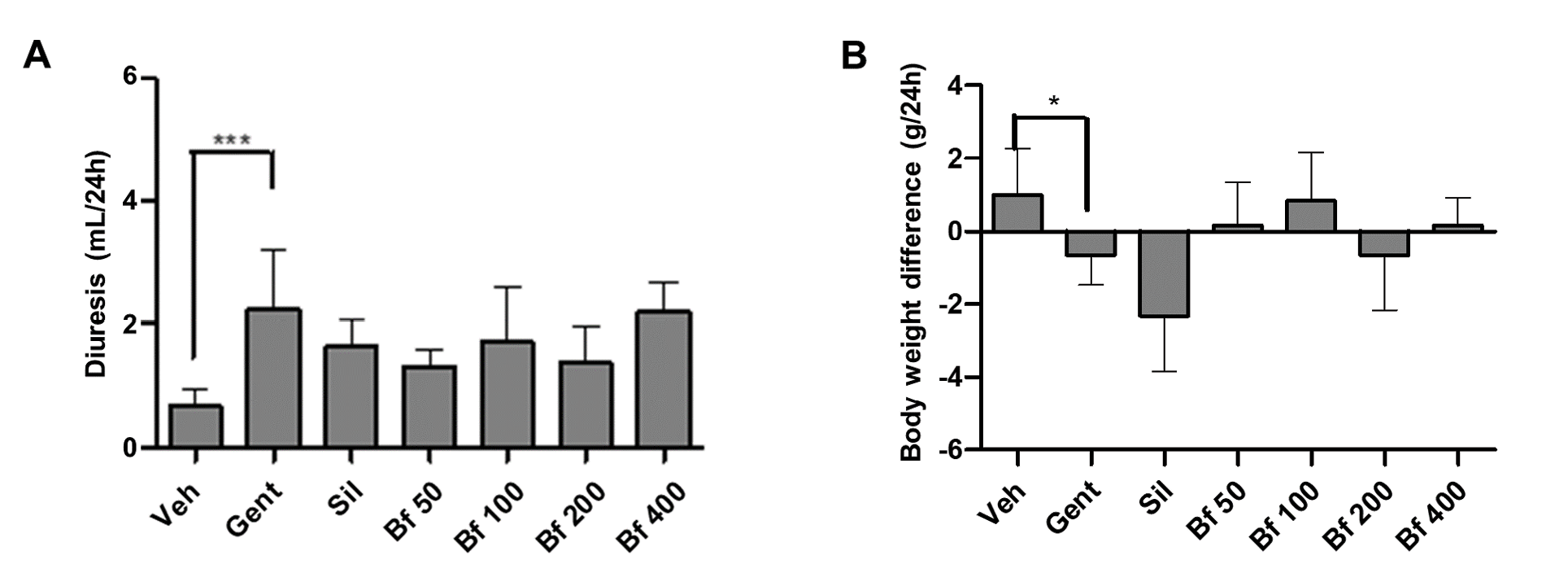
Finally, diuresis, body weight, food intake and water consumption of the animals were evaluated. A significant difference was observed between the diuresis of the pathological group (Gent) compared to the control group (Veh), as the urine volume in the Gent group was higher. The animals with kidney damage suffered a significant weight loss compared to the control group, while the animals treated with the extract (50, 100, 200, and 400 mg/kg) maintained the body weight closely related to the control group. There was no variation in terms of food or water intake by the animals (Figures 5A and 5B).
| Figure 5 - Effect of Bauhinia forficata na diurese (A) e na diferença de peso corporal (B), em ratinhos tratados com Veh: água, Gent: gentamicina, Sil: silimarina, Bf 50, Bf 100, Bf 200 e Bf 400 (B. forficata 50, 100, 200 e 400 mg/kg, p.o.). Os dados são expressos como média ± DP (n=6), ANOVA de uma via, teste post hoc de Dunnett. |
 |
Determination of hepatoprotective effect
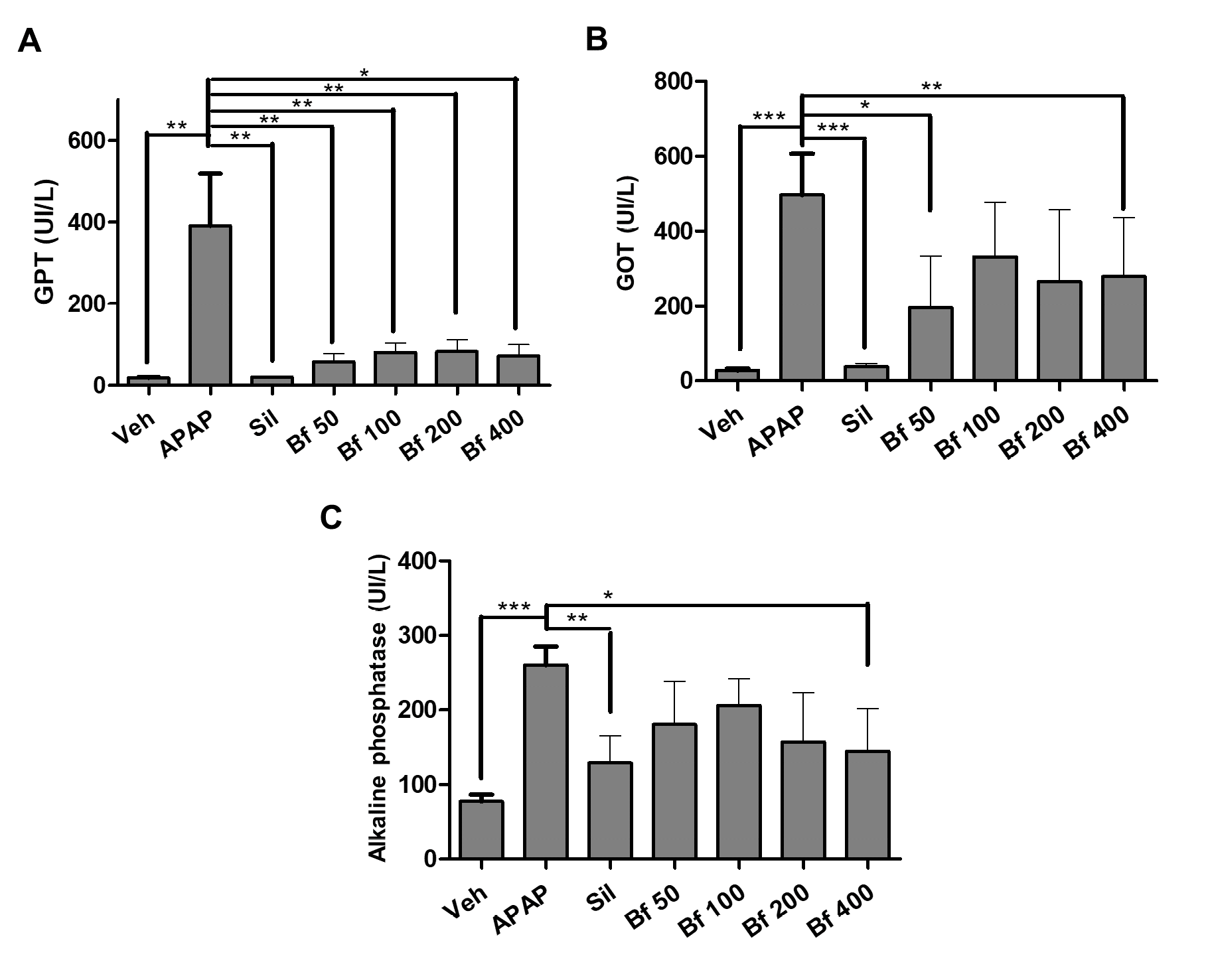
The main marker of liver damage is the activity of the enzyme GPT. A significant difference (p<0.01) was found between the control group (Veh) and the pathological group (APAP), and between the APAP group and silymarin (Sil, p<0.01). With these results, the validity of the method for this type of study was verified. B. forficata extract prevented the elevation of GPT enzyme activity, evidenced by a significant difference between GPT levels when compared to the APAP group (50, p<0.01;100, p<0.01; 200, p<0.01; 400 p<0.05 mg/kg).
Regarding the activity of the GOT enzyme, the pathological group (APAP) showed a significant difference (p<0.001) compared to the animals in control group (Veh) and the animals treated with silymarin (Sil). B. forficata extract prevented the increase in enzyme activity produced by paracetamol, moreover, 50 (p<0.05) and 400 (p<0.01) mg/kg showed significant differences when compared to the APAP group.
Finally, the activity of the enzyme alkaline phosphatase was determined. The pathological group (APAP) ALP activity was statistically different (p<0.001) from control (Veh) and silymarin (Sil, p<0.01) groups. In the groups that received the extract, the activity decreased, the difference with the APAP group was significant (p<0.05) in the group of animals treated with 400 mg/kg of B. forficata (Figures 6A-C).
| Figure 6 - Serum levels (U/l) of GOT (A), GPT (B), and Alkaline phosphatase (C) of mice (n = 6) treated with Bauhinia forficata leaves extract. Veh: water, APAP: acetaminophen, Sil: silymarin, Bf 50, Bf 100, Bf 200 and Bf 400 (B. forficata 50, 100, 200 and 400 mg/kg, p.o.). The data are plotted as average ± SD. ANOVA of one factor, followed by the Dunnett’s test was used, p<0.05 was considered statistically significant (*p<0.05; **p<0.01; *** p<0.001, significantly different from APAP). |
 |
Discussion
Medicinal plants as potential therapeutic agents are of vital importance throughout the worlds` healthcare system. Several plants demonstrated their efficacy and could be used as a therapeutic alternative to prevent and treat various liver and kidney conditions. The methanol extract of Bauhinia forficata leaves tested in this study proved to be safe at least up to 2000 mg/kg based on acute toxicity tests performed on mice, and results described previously. It has previously been reported that this plant is safe to use and does not present drug interactions (19). The nephroprotective and hepatoprotective effects were evaluated in our study using models of chemically induced kidney and liver injury in Swiss albino mice.
Aminoglycosides have nephrotoxic effects due to the induction of Reactive oxygen species (ROS) and depletion of antioxidant enzyme activities in the kidney. Gentamicin administration caused marked changes in kidney tubules due to gentamicin reabsorption in proximal convoluted tubules, causing degeneration and necrosis of the epithelial cells of the tubules. These changes were manifested by dilated tubules, loss of brush border, severe leucocyte infiltrations, tubular degeneration, and presence of tubular casts (27).
Gentamicin-induced kidney damage is mainly characterized by an increase in serum creatinine concentration, which indicates a reduction in glomerular filtration rate, and is also frequently associated with an increase in serum urea and uric acid concentration (28). Our results showed that daily administration for nine days (Gent group) increased creatinine, urea, and uric acid in serum in agreement with other results reported (28, 29). The concentration of creatinine in urine was also slightly increased, as expected (29, 30).
APAP is the most used model of intrinsic drug-induced liver injury. APAP is converted to an electrophile thought to be N-acetyl-p-benzoquinone imine (NAPQI), catalyzed by cytochrome P450 enzymes. NAPQI then binds to sulfhydryl groups on glutathione and proteins. Depletion of GSH makes the cells more susceptible to oxidative stress (31). Hepatotoxicity was achieved by intraperitoneal administration of paracetamol, 300 mg/kg body weight, used as a single administration on the last day of treatment. Liver damage was evidenced by elevated activity of liver enzymes GPT, GOT, and ALP (15-17).
Silymarin (150 mg/kg) was used in both nephroprotection and hepatoprotection assays, as previously described. The use of silymarin as a positive control in hepatoprotection models has been widely documented and validated in this type of study (15, 16, 17, 30, 32). In addition, the mechanism of the hepatoprotective effect of silymarin has been previously detailed (8). In contrast, in nephroprotection assay models, the use of silymarin to prevent kidney damage is more limited; despite this, it is still the most used option in this type of research (15, 32).
Silymarin attenuated the toxic effect of gentamicin in mice (Sil group). Some reports have indicated that silymarin has a modest nephroprotection effect against damage from nephrotoxic agents such as gentamicin (8), and its nephroprotective effect was demonstrated in previous research in our laboratory (16).
All animals treated with the doses tested of the methanolic extract of B. forficata leaves (50, 100, 200 and 400 mg/kg) prevented kidney damage caused by gentamicin, evidenced mainly by reduced creatinine levels in serum (33, 34), compared to the pathological group (Gent). Serum urea and uric acid in the groups treated with the extract was closer to the value measured in control group (Veh), as reported in the literature when nephroprotective potential of Carica papaya seeds (35) and Croton zambesicus root (36) extracts were evaluated.
Considering that the plant is popularly used for the treatment of kidney disorders, the concentrations of creatinine, urea, and uric acid in urine, together with serum and urinary sodium and potassium were also measured. It was determined that the B. forficata extract had no significant effects on these parameters (urinary creatinine, urea, and uric acid), in agreement with previous works reported on Butea monosperma and Piper cubeba (33, 34). Likewise, no difference was found between electrolyte levels, neither in serum nor in urine, as reported for Carica papaya (35) and Croton zambesicus (36). This is likely because these electrolytes are subject to complex physiological, metabolic, and hormonal regulations (35), so no significant changes have been seen in the levels of these components in the established experimental conditions. The complex regulation includes renal excretion, water intake, electrolytes balance, osmolarity, and volume of body fluids, regulated by vasopressin, aldosterone, and the effect of osmoreceptors, among others (37).
Finally, diuresis, body weight variation, food consumption, and water intake of the different test groups were also evaluated after 24 hours in metabolic cages. Diuresis augmented in Gent group, due to decreased number of functioning nephrons and increased filtration rate. Some evidence indicates that aminoglycosides interfere with the action of vasopressin on the distal nephron (38). The increased diuresis in all groups treated with the extract, compared to the control group is due to the flavonoids content, previously reported as being responsible for diuresis (19). The analysis of body weight, food, and drink has not been reported previously, it was considered that they could contribute to the enrichment of the experimental model used, providing new evidence in the pathological models used. The pathological group had lower body weight (p<0.05), and lower food and water intake (although not significant differences). Significant reduction in body weight in rats with kidney damage induced by gentamicin in a 22 day experiment were reported previously (39). In this study, differences reported correspond to the animals’ weight after 24 hours in metabolic cages.
Regarding the hepatoprotection test of the B. forficata extract, it was shown that all the doses tested (50, 100, 200 and 400 mg/kg) proved to be effective in protecting mice from liver damage caused by paracetamol. These results agree with those obtained with another species of the same genus, Bauhinia variegata (40). The presence of gallic acid and at least 39 flavonoids, such as rutin, quercetin and kaempferol has been reported in Bauhinia forficata (22, 41, 42). These compounds have antioxidant properties due to the ability to neutralize oxidizing species such as superoxide radicals. The antioxidant activity of B. forficata has been widely demonstrated and associated with flavonoid composition (19), as the activity reported in studies of an extract of Bauhinia variegata leaves (12). Hepatoprotective effect in diabetic mice was previously reported, and the effect attributed to the phenolic content (43). Moreover, the presence of trigonelline, an antioxidant alkaloid, has also been reported (44). Other species, with components in common such as Opuntia ficus indica and Zea mais, demonstrated a similar hepatoprotective effect, so it could be that the intrinsic mechanism by which these plants exert their effect is associated with the antioxidant capacity (15).
Different pharmacological activities have been reported for many Bauhinia species, including hepatoprotective and antioxidant. The leaves of Bauhinia forficate are reported to have antidiabetic, antioxidant, antibacterial, antiproliferative, antiulcerogenic, anti-inflammatory, and hepatoprotective effects in diabetic mice, among others (19). The present study was carried out to evaluate the protective activities of the methanol extract of Bauhinia forficata leaves in mice induced to acute hepatotoxicity and nephrotoxicity. These preliminary results indicate that it will be important to continue with the evaluation of the effect of this extract on other parameters that allow us to determine the antioxidant capacity, and the anti-inflammatory and anti-oxidative stress actions. More studies are required to understand mechanistic pathways of protection observed.
Conclusion
The preliminary evaluation of the methanolic extract of Bauhinia forficata leaves demonstrated moderate nephroprotective activity in mice with kidney damage induced by gentamicin, achieving significant control over the alteration of serum creatinine concentration. The extract slightly affected the serum urea and uric acid values and had very little effect on the urine parameters measured. In addition, it was shown that the extract has a marked hepatoprotective activity. Our in vivo results partially validate the popular use of B. forficata. More experiments will be required to evaluate its effects on tissues, elucidate the mechanism and identify the compounds responsible for the effect observed.
Funding sources
This research was supported by Facultad de Ciencias Químicas, and Instituto de Investigaciones en Ciencias de la Salud, from Universidad Nacional de Asunción, Paraguay. SFR conducted the work during her master's program (MCB) supported by CONACYT, PROCIENCIA Program, resources from FEEI of FONACIDE, Paraguay (PROCIENCIA POSG17-59).
Author contributions
SFR and AKG performed assays and collected data. SFR, MLK and MACB analyzed the data. PMFT, MAC-B, and MLK conceived and designed the experiments. MAC-B and MLK wrote the paper. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors would like to thank the team of Professor R. Degen, who collected and identified the plant material, Dr. N. Alvarenga, who provided the extract, and Mr. N. Kennedy for the English language revision.
Conflicts of interest
The authors declare that they have no competing interests.
References
1. Cosola., C., Sabatino., A, Di Bari., I., Fiaccadori., E., & Gesualdo., L. (2018). Nutrients, Nutraceuticals, and Xenobioti.cs Affecting Renal Health. Nutrients, 10(7), 808. https://doi.org/10.3390/nu10070808
2. Gu, X., & Manautou, J. E. (2012). Molecular mechanisms underlying chemical liver injury. Expert reviews in molecular medicine, 14, e4. https://doi.org/10.1017/S1462399411002110
3. Prikhodko., V., Bezborodkina., N. & Okovityi., S. (2022). Pharmacotherapy for Non-Alcoholic Fatty Liver Disease: Emerging Targets and Drug Candidates. Biomedicines, 10(2), 274. doi: 10.3390/biomedicines10020274
4. Instituto Nacional de Nefrología. (2019). Informe INN, Hemodiálisis Crónica. Available at: 6b307e-Informe2019INN.pdf (mspbs.gov.py)
5. Luyckx, V. A., Al-Aly, Z., Bello, A. K., Bellorin-Font, E., Carlini, R. G., Fabian, J., Garcia-Garcia, G., Iyengar, A., Sekkarie, M., van Biesen, W., Ulasi, I., Yeates, K., & Stanifer, J. (2021). Sustainable Development Goals relevant to kidney health: an update on progress. Nature reviews. Nephrology, 17(1), 15–32. https://doi.org/10.1038/s41581-020-00363-6
6. Kalra, A., Yetiskul, E., Wehrle, C. J., & Tuma, F. (2023). Physiology, Liver. In StatPearls. StatPearls Publishing.
7. Ye, F., Zhai, M., Long, J., Gong, Y., Ren, C., Zhang, D., Lin, X., & Liu, S. (2022). The burden of liver cirrhosis in mortality: Results from the global burden of disease study. Frontiers in public health, 10, 909455. https://doi.org/10.3389/fpubh.2022.909455
8. Kim., Y., Na., J., Kwon., D. & Park., J. (2018). Silymarin prevents acetaminophen-induced hepatotoxicity via up-regulation of the glutathione conjugation capacity in mice. Journal of functional food, 49, 235-240. https://doi.org/10.1016/j.jff.2018.08.025
9. Adeneye., A. & Benebo., A. (2008). Protective effect of the aqueous leaf and seed extract of Phyllanthus amarus on gentamicin and acetaminophen-induced nephrotoxic rats. Journal of Ethnopharmacology, 118(2), 318-323. https://doi.org/10.1016/j.jep.2008.04.025
10. Khan, S. A., Priyamvada, S., Farooq, N., Khan, S., Khan, M. W., & Yusufi, A. N. (2009). Protective effect of green tea extract on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Pharmacological research, 59(4), 254–262. https://doi.org/10.1016/j.phrs.2008.12.009
11. Dungca., N. (2016). Protective effect of the methanolic leaf extract of Eclipta alba (L.) Hassk. (Asteraceae) against gentamicin-induced nephrotoxicity in Sprague Dawley rats. Journal of Ethnopharmacology, 184, 18-21. doi:10.1016/j.jep.2016.03.002
12. Bashandy, S. A. E., El Awdan, S. A., Mohamed, S. M., & Omara, E. A. A. (2020). Allium porrum and Bauhinia Variegata mitigate acute liver failure and nephrotoxicity induced by thioacetamide in male rats. Indian journal of clinical biochemistry : IJCB, 35(2), 147–157. https://doi.org/10.1007/s12291-018-0803-5
13. Hina., G., Muhammad., A., Salina., S., Yazeed., A., Falak., S., Eilaf., A., Umara., A., Syed., M., Asghar., K., Muhammad., G. & Ghazala., K. (2021). Quantification of biochemical compounds in Bauhinia Variegata Linn flower extract and its hepatoprotective effect. Saudi Journal of Biological Sciences, 28(1), 247-254. https://doi.org/10.1016/j.sjbs.2020.09.056
14. Oliveira, G. O., Braga, C. P., & Fernandes, A. A. (2013). Improvement of biochemical parameters in type 1 diabetic rats after the roots aqueous extract of yacon [Smallanthus sonchifolius (Poepp.& Endl.)] treatment. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 59, 256–260. https://doi.org/10.1016/j.fct.2013.05.050
15. Ben Saad, A., Dalel, B., Rjeibi, I., Smida, A., Ncib, S., Zouari, N., & Zourgui, L. (2017). Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharmaceutical biology, 55(1), 516–525. https://doi.org/10.1080/13880209.2016.1255976
16. Soverina., M., Campuzano-Bublitz., M., Centurión., J., Galeano., A. & Kennedy., M. (2019). Preliminary evaluation of hepatoprotective and nephroprotective effects of Prosopis ruscifolia Griseb. leaves extract in mice. Journal of Applied Pharmaceutical Sciences 9(12), 37-41. doi: 10.7324/JAPS.2019.91206
17. Velázquez., A., Diarte., E., Henichen., O., Montalbetti., Y., Campuzano-Bublitz., M., Kennedy., M., Hellión-Ibarrola., M. & Ibarrola., D. (2020). Baccharis crispa attenuates toxic hepatitis induced by acetaminophen and carbon tetrachloride in mice. Journal of Applied Pharmaceutical Science 10(11), 110-116. doi: 10.7324/JAPS.2020.101115
18. Velázquez., A., Diarte., E., Galeano., A., Burgos-Edwards., A., Alvarenga., N., Heinichen., O., Montalbetti., Y., Campuzano-Bublitz., M., Kennedy., M., Hellión-Ibarrola., M. & Ibarrola., D. (2021). Hepatoprotective activity of Dorstenia brasiliensis against acute hepatitis induced by acetaminophen and carbon tetrachloride in mice, International Journal of Pharmaceutical Sciences and Research,12, 6384-6392. doi: 10.13040/IJPSR.0975-8232.12(12).6384-92
19. Cechinel-Zanchett., C., de Andrade., S. & Cechinel-Filho., V. (2018). Ethnopharmacological, Phytochemical, Pharmacological and Toxicological Aspects of Bauhinia forficata: A Mini-Review Covering the Last Five Years. Natural Product Communications 13(7), 911-916. doi:10.1177/1934578X1801300732
20. Cechinel-Zanchett., C., Melo., R., Tenfen., A., Siebert., D., Micke., G., Vitali., G., Cechinel-Filho., V., Faloni., S. & de Souza., P. (2019). Bauhinia forficata link, a Brazilian medicinal plant traditionally used to treat cardiovascular disorders, exerts endothelium-dependent and independent vasorelaxation in thoracic aorta of normotensive and hypertensive rats. Journal of Ethnopharmacology 243, 112118. https://doi.org/10.1016/j.jep.2019.112118
21. Miceli., N., Buongiorno., L., Celi., M., Cacciola., F., Dugo., P., Donato., P., Mondello., L., Bonaccorsi., I. & Taviano., M. (2016). Role of the flavonoid-rich fraction in the antioxidant and cytotoxic activities of Bauhinia forficata Link. (Fabaceae) leaves extract. Natural product research, 11, 1229-1239. doi: 10.1080/14786419.2015.1050671
22. Cechinel-Filho., V. (2009). Chemical composition and biological potential of plants from the genus Bauhinia. Phytotherapy Research 23(10), 1347-1354. https://doi.org/10.1002/ptr.2756
23. National Research Council (US). (2011). Guide for the care and use of laboratory animals. 8th ed. Washington, United States. National Academies Press (US). Available at: https://www.ncbi.nlm.nih.gov/books/NBK54050/ doi: 10.17226/12910
24. Organisation for Economic Co-operation and Development (OECD). Guidelines for the Testing of Chemicals, (2022). Oecd.org. Available at: https://www.oecd.org/chemicalsafety/testing/15487898
25. Dufour., D., Lott., J., Nolte., F., Gretch., D., Koff., R. & Seeff., L. (2000). Diagnosis and Monitoring of Hepatic Injury. I. Performance Characteristics of Laboratory Tests. Clinical Chemistry 46(12), 2027-2049. doi.org/10.1093/clinchem/46.12.2027.
26. Strömme., J. & Eldjam., L. (1974). Scandinavian Standardizations of Enzyme Determination. Scandinavian journal of clinical and laboratory investigation, 33(4), 287–289. doi: 10.1080/00365517409082498
27. Randjelovic, P., Veljkovic, S., Stojiljkovic, N., Sokolovic, D., & Ilic, I. (2017). Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI journal, 16, 388–399. https://doi.org/10.17179/excli2017-165.
28. Raju, S., Kavimani, S., Maheshwara Rao, V. U., Reddy, K. S., & Kumar, G. V. (2011). Floral extract of Tecoma stans: a potent inhibitor of gentamicin-induced nephrotoxicity in vivo. Asian Pacific journal of tropical medicine, 4(9), 680–685. https://doi.org/10.1016/S1995-7645(11)60173-9
29. Rajamurugan., R., Suyavaran., A., Selvaganabathy., N., Ramamurthy., C., Reddy., G., Sujatha., V. & Thirunavukkarasu., C. (2012). Brassica nigra plays a remedy role in hepatic and renal damage. Pharmaceutical Biology 50(12), 1488-1497. doi: 10.3109/13880209.2012.685129
30. Abdel-Kader., M., Alanazi., M., Bin., A., Al-Saikhan., F. & Hamad., A. (2017). Hepatoprotective and nephroprotective activities of Juniperus sabina L aerial. parts. Journal of Pharmacy & Pharmacognosy Research, 5(1), 29-39.
31. McGill, M. R., & Jaeschke, H. (2019). Animal models of drug-induced liver injury. Biochimica et biophysica acta. Molecular basis of disease, 1865(5), 1031–1039. https://doi.org/10.1016/j.bbadis.2018.08.037
32. Ezejiofor., A., Orish., C. & Orisakwe., O. (2014). Costus afer ker gawl leaves against gentamicin-induced nephrotoxicity in rats. Iranian Journal of Kidney Diseases. 8(4), 310-313. PMID: 25001137
33. Sonkar, N., Ganeshpurkar, A., Yadav, P., Dubey, S., Bansal, D., & Dubey, N. (2014). An experimetal evaluation of nephroprotective potential of Butea monosperma extract in albino rats. Indian journal of pharmacology, 46(1), 109–112. https://doi.org/10.4103/0253-7613.125190
34. Ahmad, Q. Z., Jahan, N., Ahmad, G., & Tajuddin (2012). Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia, 23(4), 773–781. https://doi.org/10.4103/1319-2442.98159
35. Olagunju., J., Adeneye., A., Fagbohunka., B., Bisuga., N., Ketiku., A., Benebo., A., Olufowobi., O., Adeoye., A., Alimi., M. & Adeleke., A. (2009). Nephroprotective activities of the aqueous seed extract of Carica papaya Linn. in carbon tetrachloride induced renal injured Wistar rats: a dose- and time-dependent study. Biology and Medicine 1(1):11-19.
36. Okokon, J. E., Nwafor, P. A., & Noah, K. (2011). Nephroprotective effect of Croton zambesicus root extract against gentimicin-induced kidney injury. Asian Pacific journal of tropical medicine, 4(12), 969–972. https://doi.org/10.1016/S1995-7645(11)60228-9
37. Atherton., J. (2006). Regulation of fluid and electrolyte balance by the kidney. Anaesthesia & Intensive Care Medicine 7(7), 227–233. https://doi.org/10.1053/j.mpaic.2006.04.002
38. Kaloyanides, G. J., & Pastoriza-Munoz, E. (1980). Aminoglycoside nephrotoxicity. Kidney international, 18(5), 571–582. https://doi.org/10.1038/ki.1980.175
39. Govindappa., P.K., Gautam., V, Tripathi., S.M., Sahni., Y.P., Raghavendra., H.L. (2019). Effect of Withania somnifera on gentamicin induced renal lesions in rats. Revista Brasileira de Farmacognosia 29, 234–240. https://doi.org/10.1016/j.bjp.2018.12.005
40. Bodakhe., S. & Ram., A. (2007). Hepatoprotective properties of Bauhinia variegata bark extract. Yakugaku Zasshi. Journal of the Pharmaceutical Society of Japan 127(9),1503–1507. doi: 10.1248/yakushi.127.1503
41. Silva., K. & Cechinel-Filho., V. (2002). Plantas do gênero Bauhinia: composição química e potencial farmacológico. Química Nova, 3, 449-454. https://doi.org/10.1590/S0100-40422002000300018
42. Franco, R. R., Mota Alves, V. H., Ribeiro Zabisky, L. F., Justino, A. B., Martins, M. M., Saraiva, A. L., Goulart, L. R., & Espindola, F. S. (2020). Antidiabetic potential of Bauhinia forficata Link leaves: a non-cytotoxic source of lipase and glycoside hydrolases inhibitors and molecules with antioxidant and antiglycation properties. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 123, 109798. https://doi.org/10.1016/j.biopha.2019.109798
43. Salgueiro, A. C., Folmer, V., da Silva, M. P., Mendez, A. S., Zemolin, A. P., Posser, T., Franco, J. L., Puntel, R. L., & Puntel, G. O. (2016). Effects of Bauhinia forficata Tea on Oxidative Stress and Liver Damage in Diabetic Mice. Oxidative medicine and cellular longevity, 2016, 8902954. https://doi.org/10.1155/2016/8902954
44. Toloza-Zambrano., P., Avello., M. &Fernandez., P. (2015). Determination of rutin and trigonelline in extracts of Bauhinia forficata subsp. pruinosa and hypoglycemic effect on diabetic and prediabetic patients humans. Boletín Latinoamericano y Del Caribe De Plantas Medicinales y Aromáticas, 14 (1), 21–32.
