| Original Article, Biomed Biopharm Res., 2023; 20(1):13-24 doi: 10.19277/bbr.20.1.305; PDF version [+]; Portuguese html [PT] |
Evaluation of the Lysyl Oxidase-Like 2 (LOXL2) inhibitory activity of pimaranes and their glycosyl derivatives
Sandra Ferreira 1, 4, Patrícia Rijo 1, 2 ![]() , João G. Costa 1
, João G. Costa 1 ![]() , Nuno Saraiva 1
, Nuno Saraiva 1 ![]() , Beatriz Santos 1, Clara Uriel 3, Ana María Goméz 3, A. M. Díaz-Lanza 4 & Ana S. Fernandes 1,4
, Beatriz Santos 1, Clara Uriel 3, Ana María Goméz 3, A. M. Díaz-Lanza 4 & Ana S. Fernandes 1,4 ![]()
1 - CBIOS - Center for Biosciences & Health Technologies, Universidade Lusófona de Humanidades e Tecnologias, Campo Grande 376, 1749-024 Lisboa, Portugal
2 - Instituto de Investigação do Medicamento (iMed.ULisboa), Faculdade de Farmácia, Universidade de Lisboa, 1649-003 Lisboa, Portugal
3 - Instituto de Química Orgánica, CSIC, Juan de la Cierva 3, E-28006 Madrid, Spain
4 - Universidad de Alcalá de Henares. Facultad de Farmacia, Departamento de Ciencias Biomédicas (Área de Farmacología); Ctra. Madrid-Barcelona km. 33,600 28805 Alcalá de Henares, Madrid, Spain
Abstract
Lysyl oxidase (LOX) and LOX-like 1-4 (LOXL 1-4) enzymes catalyze the cross-linking of elastin and collagen in the extracellular matrix, facilitating cell migration and invasion. The inhibition of these enzymes, particularly LOXL2, has been suggested as a therapeutic strategy to prevent breast cancer metastasis. In this work, new natural LOXL2 inhibitors were searched from Aeollanthus rydingianus, a medicinal plant rich in bioactive products. Five pimarane diterpenoids, two isolated from the plant and three derivatives, were tested. These compounds have been described for their bioactive properties such as anti-tumor, anti-inflammatory, analgesic, and antibacterial activities. In this regard, we intended to explore the mechanisms of these compounds by studying their effects on LOXL2 activity. Two pimarane diterpenoids showed a mild LOXL2 inhibitory activity as evaluated by an Amplex Ultra Red-based technique. The cytotoxicity of the most active compound was analyzed by the MTT assay in the MDA-MB-231 cell line, representative of triple-negative breast cancer. This compound decreased cell viability as single agent and increased the cytotoxic effect of doxorubicin. Its glycoconjugate was considerably more toxic, likely due to a higher uptake by cancer cells.
Keywords: breast cancer, lysyl oxidase-like 2, inhibitors, pimaranes, Aeollanthus rydingianus, MTT
To Cite: Ferreira, S., Rijo P., Costa, J. G., Saraiva N., Santos, B., Uriel, C., Goméz, A. M., Díaz-Lanza, A. M., & Fernandes, A. S. (2023) Evaluation of the Lysyl Oxidase-Like 2 (LOXL2) inhibitory activity of pimaranes and their glycosyl derivatives. Biomedical and Biopharmaceutical Research, 20(1), 13-24.
Correspondence to:
Received 10/03/2023; Accepted 14/04/2023
Introduction
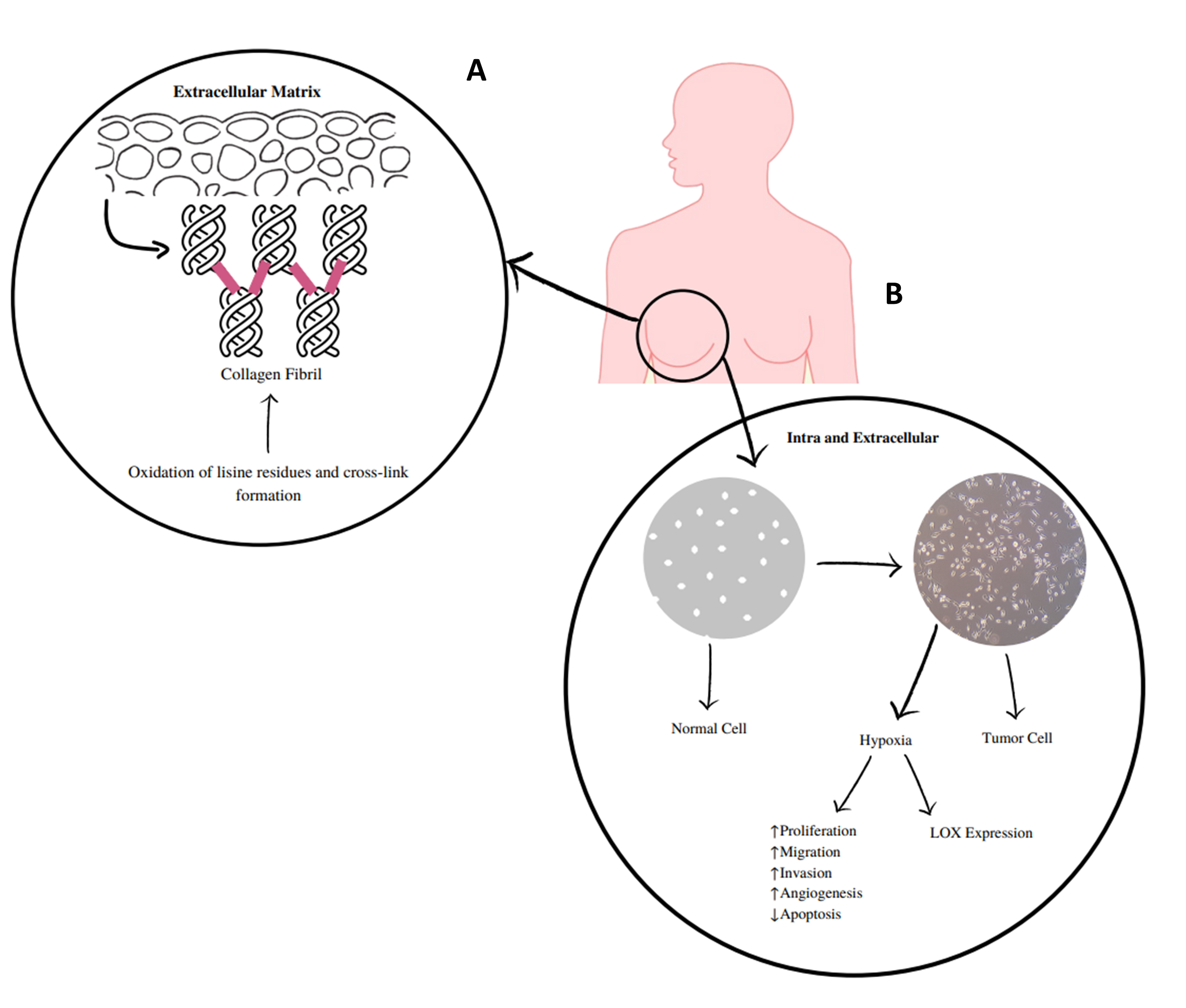
Lysyl oxidase (LOX) and lysyl oxidase-like 1 to 4 (LOXL1-LOXL4) are copper-dependent amine oxidases that covalently cross-link collagen and elastin in the extracellular matrix (ECM; Figure 1) (1–3). These LOX/LOXL proteins have been implicated in the pathogeny of several diseases, including cancer, as they are expressed in various tissues and organs (4). They serve different roles, such as regulation of gene transcription and control of cell proliferation and motility (5). The expression deregulation of these enzymes has been implicated in fibrotic processes, cancer, and neurodegenerative diseases (6–8). LOXL2 is the most studied of these enzymes and presents extracellular and intracellular activities (Figure 1) (9). Extracellularly, its overexpression promotes collagen cross-linking mediated by the deamination of lysine residues, which increases the rigidity of the ECM. Such changes in the density and rigidity of the ECM promote the invasion and progression of tumor cells through integrin modulation (9). At the intracellular level, LOXL2 can promote the activation of processes that deregulate the epithelial-to-mesenchymal transition. Some of these mechanisms are influenced by hydrogen peroxide, which is formed during the catalytic process of this enzyme and is related to the invasive effects of LOXL2 (10).
|
Figure 1 - A) LOXs catalyze the cross-linking of elastin and collagen in the extracellular matrix, which is mediated by the deamination of lysine residues and increases the stiffness of the extracellular matrix. B) Extracellular and intracellular mechanisms of LOX and LOXL 1-4. These enzymes, in particular LOXL2, are implicated in extracellular and intracellular activities. At the extracellular level, they cause changes in the density and rigidity of the extracellular matrix that promote the invasion and progression of tumor cells. Intracellularly, tumor hypoxia promotes LOXL2 upregulation, which will contribute to the deregulation of the epithelial-to-mesenchymal transition and to the pro-invasive effects of LOXL2. Some of these mechanisms are influenced by the release of hydrogen peroxide. |
 |
LOXL2 has been studied in breast cancer and particularly in triple-negative breast cancer (TNBC). This type of tumor represents 15% to 20% of breast cancer cases and is strongly related to metastatic disease (11,12). Previous studies have shown that the expression of LOX (13) and LOXL2 (14) is increased in TNBC tumors. The expression of LOX and LOXL1-4 has also been studied in the representative TNBC human cell line MDA-MB-23, suggesting that the LOX and LOXL2 enzymes have the strongest association with an invasive/metastatic phenotype (15). A bioinformatics analysis made by our group showed that LOXL2 expression is associated with lower disease-free survival in breast cancer patients, particularly in the triple-negative and luminal A subtypes (16). Moreover, in TNBC, LOXL2 expression correlates with increased cancer-associated fibroblasts and endothelial cells and with decreased lymphocyte infiltrates, which are also characteristics of a poorer prognosis (16). These aspects, along with the scarce therapeutic options currently available, make TNBC a suitable candidate to benefit from LOXL2 inhibition.
Since antiquity, plants have been used as sources of compounds with therapeutic activity. In fact, the first LOX inhibitor known was β-aminopropionitrile (BAPN) is a phytochemical compound present in sweet pea (Lathyrus odoratus L.) (17,18). The current knowledge and available technology can greatly contribute to the discovery of new drugs obtained from traditional plants.
In this work, pimarane-type compounds isolated from Aeollanthus rydingianus (Lamiaceae) or their derivatives were screened as potential inhibitors of the human LOXL2 (hLOXL2). The genus Aeollanthus Mart. ex Spreng. (Labiatae) comprises over 100 species and occurs mainly in southern Africa and Brazil. Plants of this genus contain compounds with interesting biological activities, including antimicrobial, antifungal and anticonvulsant properties (19). The species studied herein, Aeollanthus rydingianus Van Jaarsv. and A.E. van Wyk, grows in northern Namibia and southern Angola in soils that are relatively moist and acidic (20). From the aerial parts of this plant, different pimarane compounds have been isolated (19). These compounds have been described for their therapeutic properties, including antitumor, anti-inflammatory, analgesic, and antibacterial activities (19,21). Glycosidic derivatives were included as it has been reported/shown that linking a glycoside moiety to a given drug can improve its efficacy and selectivity for cancer cells. Due to their higher metabolic rate, the uptake of glycosylated drugs, likely mediated by glucose transport proteins, is much higher in cancer cells than in normal ones. In this context, the glycosylation of bioactive natural compounds may provide tumor-targeting properties, minimizing potentially deleterious systemic effects (22).
Material and methods
Chemicals
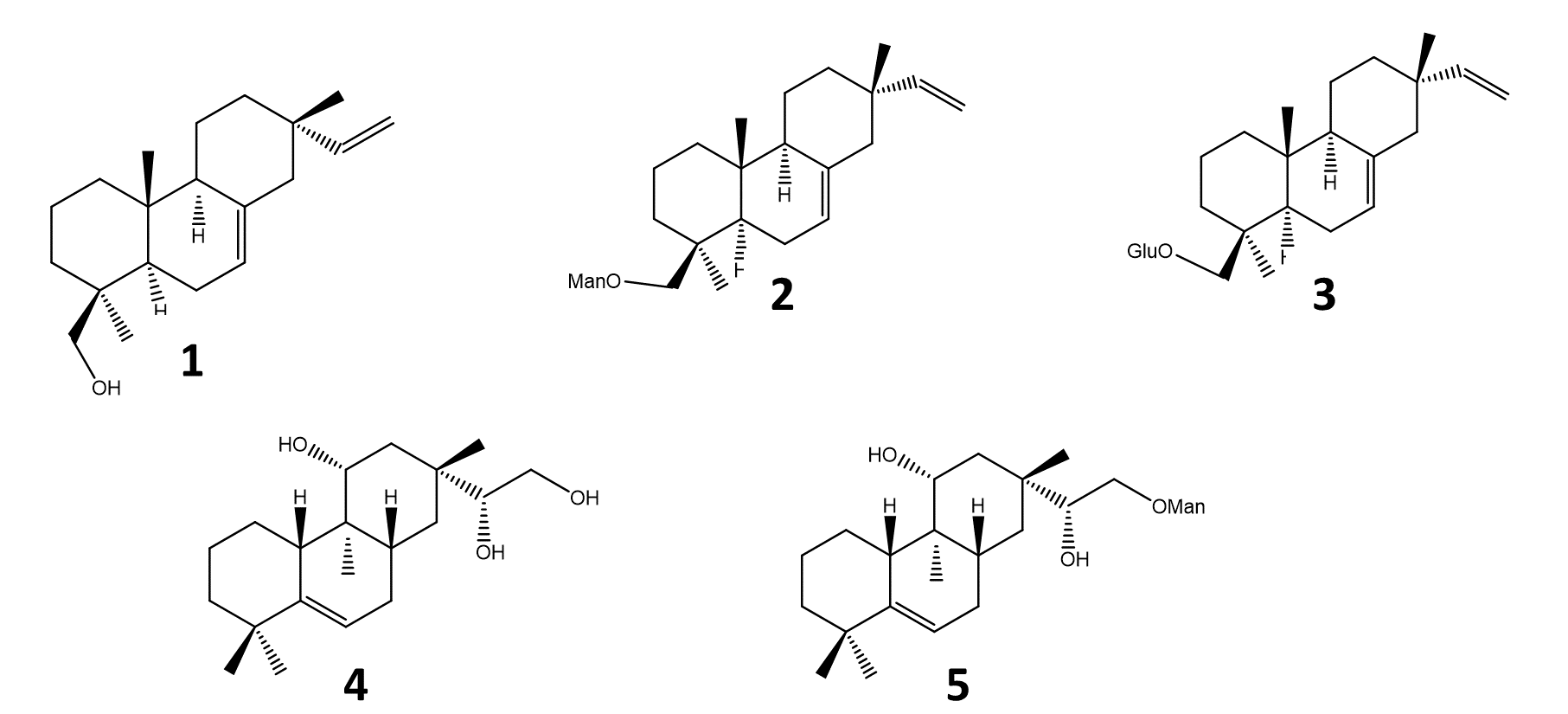
Five pimarane compounds (Figure 2), 7, 15-isopimaradien-19-ol [1], O-β-D-mano-copyranoside-7,15-isopimaradiene [2], 19-O-β-D-glucopyranoside-7,15-isopimaradiene [3], lagascatriol [4] and lagascatriol-16-O-α-D-mannopyranoside [5] (19,21) were obtained as previously described (21). Human LOXL2 was acquired from Sino Biological (Beijing, China). Amplex Ultra Red (AUR), sodium borate and BAPN were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Urea was obtained from VWR International (Darmstadt, Germany). Sodium chloride was acquired from José M. Vaz Pereira, S.A (Benavente, Portugal). Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum (FBS) were purchased from Biowest (Nuaillé, France). Horseradish peroxidase (HRP), penicillin-streptomycin solution, trypsin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Dimethylsulfoxide (DMSO), ethanol, and doxorubicin were obtained from Merck (Darmstadt, Germany). Matrigel™ was purchased from BD Biosciences (San Jose, CA, USA).
|
Figure 2 - Pimaranes diterpenes isolated from Aeollanthus rydingianus (1 and 4) and their derivatives (2, 3 and 5). [1] 7, 15-isopimaradien-19-ol, [2] 19-O-α-D-mannopyranoside-7,15-isopimaradiene, [3] 19-O-β-D-glucopyranoside-7,15-isopimaradiene; [4] lagascatriol; [5] lagascatriol-16-O-α-D-mannopyranoside. |
 |
Biochemical assay
The activity of human hLOXL2 was determined by measuring the hydrogen peroxide released during the reaction using the AUR reagent in the presence of HRP (23,24). Compounds and hLOXL2 (10 nM) were added to Assay Buffer (0.05 M sodium borate, 1.2 M urea, and 0.01 M sodium chloride at pH 8.0) in black-wall 96-well optical plates. After 15 minutes of incubation at 37ºC, cadaverine (0.5 mM), AUR (50 µM) and HRP (0.5 UmL-1) were added. The fluorescence was measured on a BioTek Synergy HTX, using an excitation wavelength of 563 nm and emission of 587 nm over 50-minute kinetics. The rate of the reaction was obtained from the slope of the linear portion of the read. The method was validated using BAPN as positive control (24).
Cell culture
The human breast cancer cell line MDA-MB-231 was obtained from ATCC. Cells were kept in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 0.1 mg/ mL streptomycin. Cultures were incubated at 37 °C, under a humidified atmosphere containing 5% CO2 in air.
Cell viability assay
The effect of the pimaranes on cell viability, either individually or in combination with doxorubicin, was determined by the MTT assay. Briefly, 6 × 103 cells, were cultured in 200 μL of a complete medium in 96-well plates. The cells were grown for 24 h and then exposed to different concentrations of the pimaranes and/or doxorubicin (1 and 5 μM), for a 24 h or 48 h period. The MTT reduction assay was performed as previously described (25). Three to seven independent experiments were performed, and five replicate cultures were used for each condition. The IC50 values were calculated using GraphPad software (version 8), using the logarithm of the concentrations versus the percentage of cell viability.
Statistical Analysis
Differences in mean values of the results were evaluated by Student's t-test, after assessing the normality and the homogeneity of the variances of continuous variables. The analyses were performed with SPSS statistical package (version 25, SPSS Inc. Chicago, IL, USA).
Results
The five compounds studied in this work were screened for their ability to inhibit hLOXL2, using the Amplex Ultra Red methodology. The obtained results are shown in Table 1. The assay was first validated with BAPN, as it has been previously described as a LOXL2 inhibitor. An IC50 value of 228.1 nM was obtained for BAPN, which is in accordance with the values reported in the literature (24).
|
Table 1 - IC50 values for the inhibition of hLOXL2, obtained by the Amplex Ultra Red technique. BAPN - β-aminopropionitrile; ND - Not determined. |
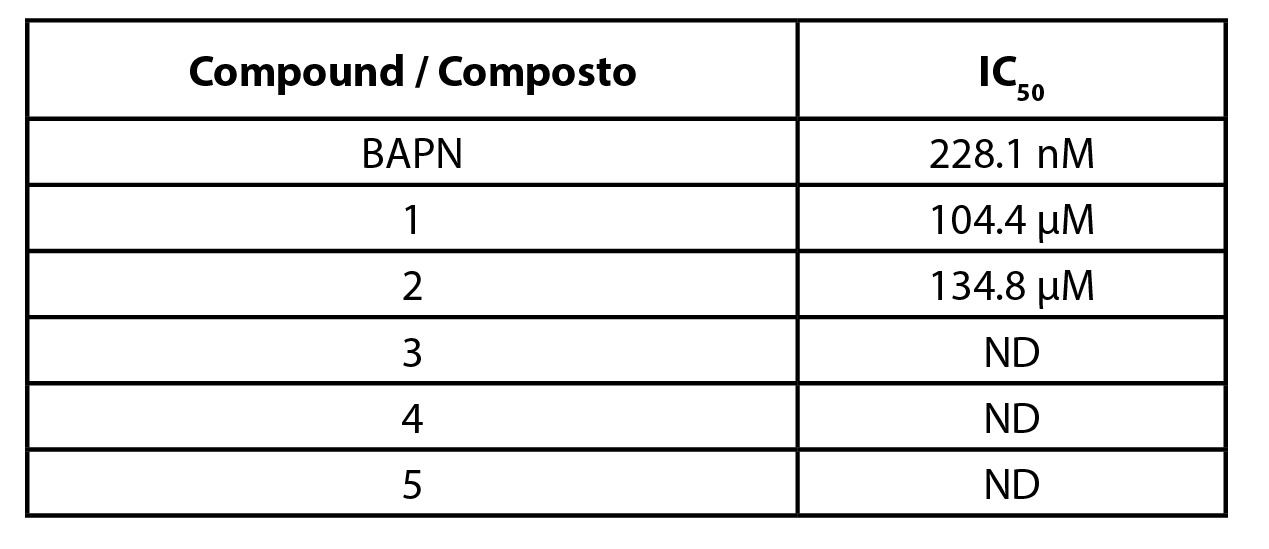 |
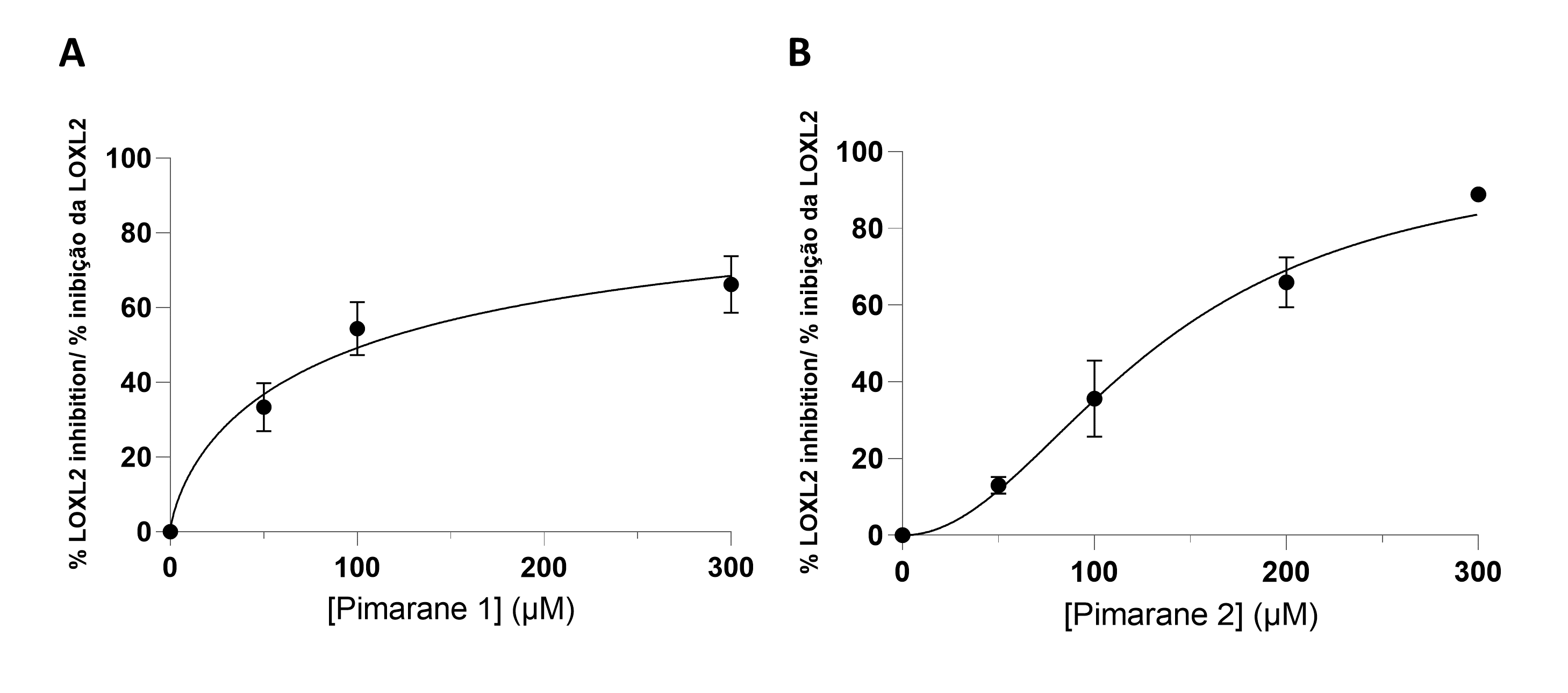
All pimaranes were initially tested at a concentration of 100 µM. The naturally occurring pimarane 1 and its mannose derivative 2 were able to inhibit hLOXL2. The non-glycosilated form had a higher activity. The respective concentration-response curves are shown in Figure 3. Both compounds exhibited IC50 values in the micromolar range: 104 µM for compound 1 and 134 µM for compound 2 (Table 1). Contrarily to compound 1 and its mannose derivative (2), the glucose conjugate of compound 1 (pimarane 3) did not show hLOXL2 inhibitory activity at the 100 µM concentration. The same lack of activity was observed for the natural pimarane 4 and its mannose derivative 5.
|
Figure 3 - LOXL2 inhibition assay, according to the Amplex Ultra Red technique. A) Pimarane 1. B) Pimarane 2. |
 |
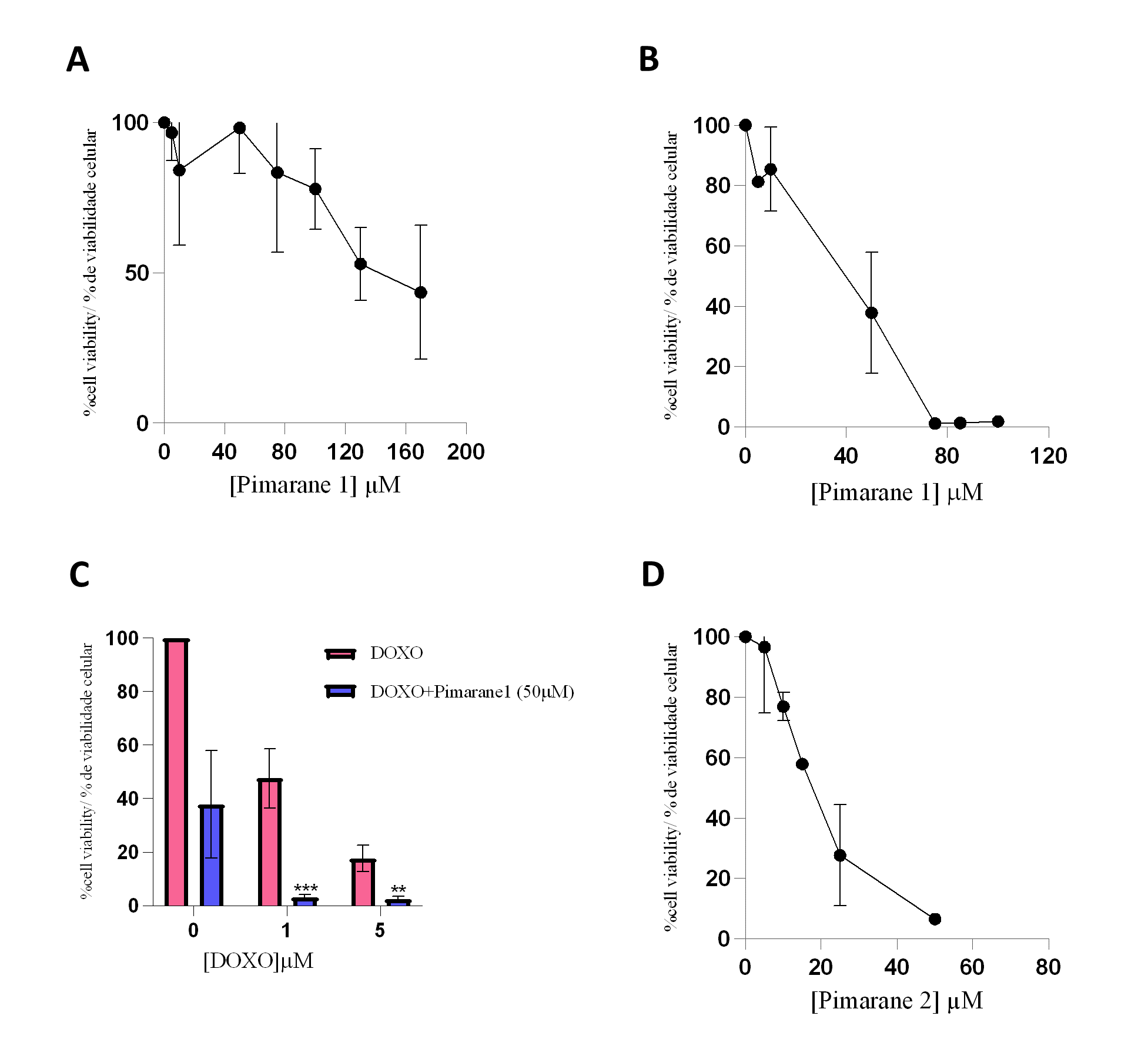
Pimaranes 1 and 2, which showed LOXL2 inhibitory activity, were further studied to characterize their impact on cell viability. The cytotoxicity study was carried out in the MDA-MB-231 cell line, using the MTT assay, after treatment periods of 24 and 48 hours as described in the previous section. The concentrations of pimarane compounds 1 and 2 were chosen to allow for the definition of the cytotoxicity profile, i.e., from non-toxic to highly toxic concentrations. However, in the case of 24 hour incubation with compound 1, the limited solubility of the compound precluded studies with higher concentrations.
Pimarane 1 exerted cytotoxic effects that were concentration- and time-dependent, as depicted in Figures 4A and 4B. The IC50 values obtained for this compound were 113.4 µM (24 h) and 49.5 µM (48 h). Since the inhibition of LOXL2 has been suggested as a strategy to sensitize TNBC cells to conventional therapy (26), the cytotoxicity of pimarane 1 (50 µM) in combination with doxorubicin (1 and 5 µM) was also evaluated (Figure 4C). As expected, doxorubicin alone decreased the cell viability in a concentration-dependent manner. Moreover, when pimarane 1 was given in combination with doxorubicin, the cytotoxicity was much more pronounced as a consequence of a possible additive effect. The difference in the viability of the cells that received the combined treatment when compared to the viability of the cells only exposed to doxorrubicin was statistically significant for both concentrations of doxorubicin (p < 0.01). Pimarane 2 also decreased the viability of MDA-MB-231 cells in a concentration-dependent manner (Figure 4 D), with an IC50 value of 16.6 µM for 24 hour treatment. Comparing both pimaranes, it is clear that pimarane 2 is considerably more toxic than pimarane 1.
|
Figure 4 - Impact of pimaranes 1 and 2 in the viability of MDA-MD-231 cells, evaluated by the MTT assay. A) Pimarane 1, 24 hours incubation. B) Pimarane 1, 48 hours incubation. C) Co-incubation of pimarane 1 (50 µM) and doxorubicin, 48 hours incubation; *** p < 0.001 and ** p < 0.01 when compared with doxorubicin-exposed cells. D) Pimarane 2, 24 hours incubation. |
 |
Discussion and conclusions
LOXL2 inhibitors have been suggested as a promising treatment to prevent metastasis and invasion of breast cancer (3). Among the different breast cancer subtypes, TNBC seems particularly relevant in this context.
A screening of some pimarane diterpenoid compounds with the abietane skeleton, obtained from a Lameacea plant, was conducted. From the fives compounds analyzed, only pimaranes 1 and 2 demonstrated inhibition of LOXL2 activity. However, although these two compounds inhibited LOXL2, they presented higher IC50 values when compared to the positive control BAPN or to synthetic LOXL2 inhibitors previously reported in the literature (27–29). The concentrations of pimarane compounds 1 and 2 required for an efficient LOXL2 inhibition may be difficult to achieve in an eventual clinical context.
For the cytotoxicity assay, the cell line MDA-MB-231 was chosen. These cells are commonly used as an in vitro model of TNBC since they are highly aggressive, invasive, and poorly differentiated, and lack oestrogen and progesterone receptors expression, as well as HER2 (human epidermal growth factor receptor 2) amplification. Furthermore, these cells express high levels of LOXL2, and their invasiveness is mediated by proteolytic degradation of the extracellular matrix. For the combinatory assay, the anticancer drug doxorubicin was used, as it is widely used in chemotherapy regimens for metastatic breast cancer (25). Both pimaranes 1 and 2 exhibited considerable cytotoxicity in MDA-MB-231 cells at concentrations below those at which LOXL2 activity is inhibited. These data suggest that although the compounds could be further explored as potential anticancer drugs, their interest as LOXL2 inhibitors is limited. In addition, mechanisms unrelated to the inhibition of LOXL2 are likely involved in the cytotoxic effects observed. Likewise, the enhancement of cytotoxicity observed when cells were simultaneously exposed to pimarane 1 and doxorubicin is probably not related to LOXL2 inhibition but ascribed to other mechanisms.
When comparing the cytotoxicity of pimarane compounds 1 and 2, it is noticeable that pimarane 2 is ~7-fold more toxic than pimarane 1. The structural difference between these compounds relies on the mannose group. The presence of the sugar may increase the uptake of glycoconjugates by cancer cells, leading to more pronounced cytotoxic effects. Due to the Warburg effect, cancerous tissues consume larger amounts of glucose compared to normal tissue. The glucose transporter GLUT-1 is widely overexpressed in a large percentage of human cancers, including breast cancer (30). Therefore, conjugation of anticancer agents to sugars takes advantage of GLUT-mediated cellular entry for preferential delivery to cancer cells, improving efficacy and selectivity (30). This fact may justify the differential cytotoxicity observed for pimaranes 1 and 2.
The strategy presented here could be applied to screen other natural compounds as potential LOXL2 inhibitors. Although the compounds studied in this work are not promising as novel inhibitors of LOXL2, pimaranes 1 and 2 could be useful as a starting point for structural optimization towards the design of more potent inhibitors, with potential therapeutic interest. Furthermore, our results corroborate the idea that linking a sugar moiety to a given anticancer drug could constitute a targeting strategy to increase the efficacy of anticancer drugs against breast cancer.
Author Contributions
Conceptualization, A.S.F. and P.R.; investigation and data analysis, S.F, J.G.C., N.S., C.U., A.M.G., D.L.A.M., A.S.F. and P.R.; writing -original draft preparation, S.F; writing - review and editing, A.S.F., J.G.C. and PR; supervision, A.S.F.; project administration, A.S.F.; funding acquisition, A.S.F. and P.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by national funds through FCT-Foundation for Science and Technology, I.P., under the UIDB/04567/2020 and UIDP/04567/2020 projects. Research developed with funding from Universidade Lusófona/ILIND (Grant Programmes FIPID 2019/2020 and Fazer+ ILIND/F+/EI/01/2020).
Conflicts of Interest
The authors declare no conflict of interest. The editors involved in this manuscript's authorship had no participation in the review or decision process. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
1. Rucker, R. B., Kosonen, T., Clegg, M. S., Mitchell, A. E., Rucker, B. R., Uriu-Hare, J. Y., & Keen, C. L. (1998). Copper, lysyl oxidase, and extracellular matrix protein cross-linking. The American journal of clinical nutrition, 67(5 Suppl), 996S–1002S. https://doi.org/10.1093/ajcn/67.5.996S
2. Molnar J., Fong K.S.K., He Q.P., Hayashi K., Kim Y., Fong S.F.T. et al. (2003). Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1647(1–2), 220–4. https://doi.org/10.1016/S1570-9639(03)00053-0
3. Ferreira S., Saraiva N., Rijo P., and Fernandes A.S. (2021). LOXL2 Inhibitors and Breast Cancer Progression. Antioxidants, 10(2), 312. https://doi.org/10.3390/antiox10020312
4. Hayashi, K., Fong, K. S., Mercier, F., Boyd, C. D., Csiszar, K., & Hayashi, M. (2004). Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. Journal of molecular histology, 35(8-9), 845–855. https://doi.org/10.1007/s10735-004-2340-1
5. Barker, H. E., Cox, T. R., & Erler, J. T. (2012). The rationale for targeting the LOX family in cancer. Nature reviews. Cancer, 12(8), 540–552. https://doi.org/10.1038/nrc3319
6. Barry-Hamilton, V., Spangler, R., Marshall, D., McCauley, S., Rodriguez, H. M., Oyasu, M., Mikels, A., Vaysberg, M., Ghermazien, H., Wai, C., Garcia, C. A., Velayo, A. C., Jorgensen, B., Biermann, D., Tsai, D., Green, J., Zaffryar-Eilot, S., Holzer, A., Ogg, S., Thai, D., … Smith, V. (2010). Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nature medicine, 16(9), 1009–1017. https://doi.org/10.1038/nm.2208
7. Yeung, T., Georges, P. C., Flanagan, L. A., Marg, B., Ortiz, M., Funaki, M., Zahir, N., Ming, W., Weaver, V., & Janmey, P. A. (2005). Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell motility and the cytoskeleton, 60(1), 24–34. https://doi.org/10.1002/cm.20041
8. Kumari S., Panda T.K., and Pradhan T. (2017). Lysyl Oxidase: Its Diversity in Health and Diseases. Indian Journal of Clinical Biochemistry, 32(2), 134–41. https://doi.org/10.1007/s12291-016-0576-7
9. Setargew Y.F.I., Wyllie K., Grant R.D., Chitty J.L., and Cox T.R. (2021). Targeting lysyl oxidase family meditated matrix cross‐linking as an anti‐stromal therapy in solid tumours. Cancers, 13(3), 491. https://doi.org/10.3390/cancers13030491
10. Wang W., Wang X., Yao F., and Huang C. (2022). Lysyl Oxidase Family Proteins: Prospective Therapeutic Targets in Cancer. International journal of molecular sciences, 23(20), 12270. https://doi.org/10.3390/ijms232012270
11. Zagami, P., & Carey, L. A. (2022). Triple negative breast cancer: Pitfalls and progress. NPJ breast cancer, 8(1), 95. https://doi.org/10.1038/s41523-022-00468-0
12. Luo C., Wang P., He S., Zhu J., Shi Y., and Wang J. (2022). Progress and Prospect of Immunotherapy for Triple-Negative Breast Cancer. Frontiers in Oncology, 12, 919072. https://doi.org/10.3389/fonc.2022.919072
13. Leo, C., Cotic, C., Pomp, V., Fink, D., & Varga, Z. (2018). Overexpression of Lox in triple-negative breast cancer. Annals of diagnostic pathology, 34, 98–102. https://doi.org/10.1016/j.anndiagpath.2018.03.009
14. Ahn, S. G., Dong, S. M., Oshima, A., Kim, W. H., Lee, H. M., Lee, S. A., Kwon, S. H., Lee, J. H., Lee, J. M., Jeong, J., Lee, H. D., & Green, J. E. (2013). LOXL2 expression is associated with invasiveness and negatively influences survival in breast cancer patients. Breast cancer research and treatment, 141(1), 89–99. https://doi.org/10.1007/s10549-013-2662-3
15. Molani Gol R., and Kheirouri S. (2021). The Effects of Quercetin on the Apoptosis of Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231: A Systematic Review. Nutrition and Cancer, 74(2), 405–22. 10.1080/01635581.2021.1897631
16. Ramos, S., Ferreira, S., Fernandes, A. S., & Saraiva, N. (2022). Lysyl Oxidases Expression and Breast Cancer Progression: A Bioinformatic Analysis. Frontiers in pharmacology, 13, 883998. https://doi.org/10.3389/fphar.2022.883998
17. Sherif H. M. (2010). In search of a new therapeutic target for the treatment of genetically triggered thoracic aortic aneurysms and cardiovascular conditions: insights from human and animal lathyrism. Interactive cardiovascular and thoracic surgery, 11(3), 271–276. https://doi.org/10.1510/icvts.2010.239681
18. Schilling E.D., Strong F.M. (1955). Isolation, Structure and Synthesis of a Lathyrus Factor from L. Odoratus. Journal of the American Chemical Society, 77, 2843–2845.
19. Rijo P., Simões M.F., Duarte A., and Rodríguez B. (2009). Isopimarane diterpenoids from Aeollanthus rydingianus and their antimicrobial activity. Phytochemistry, 70(9), 1161–5. https://doi.org/10.1016/J.PHYTOCHEM.2009.06.008
20. van Jaarsveld A.E.J., and van Wyk A.E. (2005). Notes on african plants The Aeollanthus abyssinicus group (Labiatae). Bothalia 35(2), 157-160. https://doi.org/10.4102/abc.v35i2.391
21. Isca V.M.S., Andrade J., Fernandes A.S., Paixão P., Uriel C., Gómez A.M. et al. (2020). In Vitro Antimicrobial Activity of Isopimarane-Type Diterpenoids. Molecules, 25(18), 4250. https://doi.org/10.3390/molecules25184250
22. Sorg B.L., Hull W.E., Kliem H.C., Mier W., and Wiessler M. (2005). Synthesis and NMR characterization of hydroxyurea and mesylglycol glycoconjugates as drug candidates for targeted cancer chemotherapy. Carbohydrate Research 340(2), 181–9. https://doi.org/10.1016/J.CARRES.2004.11.024
23. Guerreiro, Í., Vidovic, B., Costa, J. G., Martins, M., Ferreira, S., Oliveira, N. G., Saraiva, N., & Fernandes, A. S. (2023). The Dietary Isothiocyanate Erucin Reduces Kidney Cell Motility by Disturbing Tubulin Polymerization. Molecular nutrition & food research, 67(3), e2200581. https://doi.org/10.1002/mnfr.202200581
24. Hutchinson, J. H., Rowbottom, M. W., Lonergan, D., Darlington, J., Prodanovich, P., King, C. D., Evans, J. F., & Bain, G. (2017). Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX. ACS medicinal chemistry letters, 8(4), 423–427. https://doi.org/10.1021/acsmedchemlett.7b00014
25. Flórido, A., Saraiva, N., Cerqueira, S., Almeida, N., Parsons, M., Batinic-Haberle, I., Miranda, J. P., Costa, J. G., Carrara, G., Castro, M., Oliveira, N. G., & Fernandes, A. S. (2019). The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells. Redox biology, 20, 367–378. https://doi.org/10.1016/j.redox.2018.10.016
26. Cebrià-Costa J.P., Pascual-Reguant L., Gonzalez-Perez A., Serra-Bardenys G., Querol J., Cosín M. et al. (2020). LOXL2-mediated H3K4 oxidation reduces chromatin accessibility in triple-negative breast cancer cells. Oncogene, 39(1), 79–121. https://doi.org/10.1038/s41388-019-0969-1
27. Smithen, D. A., Leung, L. M. H., Challinor, M., Lawrence, R., Tang, H., Niculescu-Duvaz, D., Pearce, S. P., Mcleary, R., Lopes, F., Aljarah, M., Brown, M., Johnson, L., Thomson, G., Marais, R., & Springer, C. (2020). 2-Aminomethylene-5-sulfonylthiazole Inhibitors of Lysyl Oxidase (LOX) and LOXL2 Show Significant Efficacy in Delaying Tumor Growth. Journal of medicinal chemistry, 63(5), 2308–2324. https://doi.org/10.1021/acs.jmedchem.9b01112
28. Leung L., Niculescu-Duvaz D., Smithen D., Lopes F., Callens C., McLeary R. et al. (2019). Anti-metastatic Inhibitors of Lysyl Oxidase (LOX): Design and Structure-Activity Relationships. Journal of medicinal chemistry, 62(12), 5863–84. https://doi.org/10.1021/acs.jmedchem.9b00335
29. Rowbottom M.W., Bain G., Calderon I., Lasof T., Lonergan D., Lai A. et al. (2017). Identification of 4-(Aminomethyl)-6-(trifluoromethyl)-2-(phenoxy)pyridine Derivatives as Potent, Selective, and Orally Efficacious Inhibitors of the Copper-Dependent Amine Oxidase, Lysyl Oxidase-Like 2 (LOXL2).Journal of medicinal chemistry, 60 (10), 4403–23. https://doi.org/10.1021/acs.jmedchem.7b00345
30. Calvaresi, E. C., & Hergenrother, P. J. (2013). Glucose conjugation for the specific targeting and treatment of cancer. Chemical science, 4(6), 2319–2333. https://doi.org/10.1039/C3SC22205E
