| Original Article, Biomed Biopharm Res., 2023; 20(2):58-73 doi: 10.19277/bbr.20.2.329; Bilingual PDF [+]; Portuguese html [PT] |
Antimicrobial activity of nanoparticles prepared with
O-carboxymethylchitosan:γ-Fe2O3:silver and extract of Piper solmsianum
Francieli Molinett 1 ![]() , Clovis Antonio Rodrigues 1
, Clovis Antonio Rodrigues 1 ![]() , Gizelle Inácio Almerindo 1
, Gizelle Inácio Almerindo 1 ![]() , Rosi Zanoni da Silva 2
, Rosi Zanoni da Silva 2 ![]() , Jacir Dal Magro 3
, Jacir Dal Magro 3 ![]() , Rafael Martello 3
, Rafael Martello 3 ![]() , Anna Slawska-Waniewska 4
, Anna Slawska-Waniewska 4 ![]() , Natalia Nedelko 4
, Natalia Nedelko 4 ![]() , & Alexandre Bella Cruz 1
, & Alexandre Bella Cruz 1 ![]()
1 - Núcleo de Investigações Químico-Farmacêuticas (NIQFAR), Universidade do Vale do Itajaí (UNIVALI), Itajaí, SC, Brazil, 88.302-202. Fax +55 (47) 3341.7601
2 - Departamento de Ciências Farmacêuticas, Universidade Estadual de Ponta Grossa (UEPG), Av. Carlos Cavalcanti, 4748, Uvaranas, Ponta Grossa, PR, Brazil, 84.030-900
3 - Programa de Pós-Graduação em Ciências Ambientais, Universidade Comunitária da Região de Chapecó (UNOCHAPECÓ), Servidão Anjo da Guarda, 295-D, Bairro Efapi, Chapecó, SC, Brazil, 89.809-900
4 - Institute of Physics, Polish Academy of Sciences, Aleja Lotników 32/46 PL–02668, Warsaw, Poland
Abstract
Silver nanoparticles incorporated into magnetic nanocomposite were synthesized using a crude methanolic extract from Piper solmsianum, which was used as an agent for reduction and stabilization in phytochemical-assisted synthetic nanomaterial. The antimicrobial activity of the pathogenic microorganisms is reported here. The synthesized nanoparticles were also characterized by transmission electron microscopy and quantification of Ag analysis. The antimicrobial activity was evaluated by the broth microdilution method, against the bacteria Bacillus subtilis, Staphylococcus aureus and Escherichia coli, and the yeast Candida albicans. Among the nine compounds tested, CMAgPs2 and CMAgPs5 presented the best results in terms of activity against bacteria (bactericidal) and yeast (fungistatic). It was observed that crude methanolic extract proved to be a potential reducing agent for green synthesis. It was also found that no CMAgPs sample was toxic in the Artemia salina test.
Keywords: Silver nanoparticle, magnetic, Piper, antimicrobial, carboxymethylchitosan
To Cite: Molinett, F., et al. (2023) Antimicrobial activity of nanoparticles prepared with O-carboxymethylchitosan:γ-Fe2O3:silver and extract of Piper solmsianum. Biomedical and Biopharmaceutical Research, 20(2), 58-73.
Author correspondence:
Received: 16/11/2023; Accepted: 31/12/2023
Introduction
Silver nanoparticles (NPs) are widely used in nanotechnology. Due to their special physical-chemical properties, silver NPs are frequently applied in numerous areas, including medicine, health, agriculture, packaging, and electronics. One of the main problems in using NPs in chemical reactions is related to the agglomeration of NPs. The immobilization of NPs to solid supports, such as polymers (1), carbonaceous materials (2), and metal oxide (3), prevents the agglomeration of NPs during chemical reactions.
The use of plants or plant extracts in the green synthesis of silver nanoparticles has advantages over other biological resources because plants are found everywhere. Different parts of the plants were used for synthesis, such as leaves, roots, fruits, stems, bark, and seeds. Different extracts, such as aqueous, methanolic, hydroalcoholic, ethanolic extracts, are the most used for this purpose (4). The presence of secondary metabolites in plants, and consequently in extracts such as terpenoids, flavonoids and alkaloids, leads to the reduction and capping of nanoparticles (5-9).
According to the World Health Organization, around 2 billion people around the world use water from sources contaminated with feces, which can transmit diseases such as diarrhea, cholera, dysentery, typhoid and poliomyelitis. In addition, 844 million people do not have access to safe drinking water (10). Water treatment technologies are urgently needed, especially point-of-use (POU) disinfection technologies that are suitable for situations where there are limited resources. As such, the POU's water treatment technologies have received widespread attention worldwide (11).
Magnetic nanoparticles (MNPs) are classes of nanoparticles that generally contain iron (Fe0, Fe3O4, γ-Fe2O3). Due to their magnetic properties, the particles can be easily separated from the processing media by applying an external magnetic field. MNPs have broad application in different areas, but especially in those related to the environment (12).
In this aspect, when prepared or incorporated with substances containing antimicrobial or adsorbent properties, MNPs can be employed for the purification of water in POU systems.
Piper solmsianum C. DC. (sin. Piper leucanthum or Piper santosanum), commonly known as caapeba or pariparoba in Brazil, is a shrub that is frequently found in areas with wet tropical soils. It measures 1 to 3 m in height and presents glabrous and striated branches. Its leaves are petiolate and the blade is ovate with an acuminate apex and truncated to a cordate base. It presents hermaphroditic flowers represented by spike-like inflorescences (13-15).
Phytochemical studies of the plant have indicated the presence of aliphatic hydrocarbons, monoterpenes, sesquiterpenes, arylpropanoids, neolignans (15-17) and flavonoids (18). It has been reported that the plant has antibacterial (19), anti-tuberculosis (20), antifungal (18), and trypanocidal (21) activities.
In this in vitro study, we focused on the antimicrobial properties of O-carboxymethychitosan/γ-Fe2O3/Ag0/P. solmsianum extract (CMAgPs). First, the nanoparticles were synthesized and characterized. Then, the antimicrobial properties of the CMAgPs were tested on the microorganisms (Staphylococcus aureus, Escherichia coli, Candida albicans, Bacillus subtilis). The effects of this nanoparticle on the living organism Artemia salina were evaluated.
Materials and methods
The nanoparticles O-carboxymethylchitosan/γ-Fe2O3 (CM) and γ-Fe2O3 (MNP) were synthesized and characterized (1). Edds of Artemia salina (high hatching) were purchased from Maramar Aquacultura Com. Imp. Exp. Ltda (Rio de Janeiro, Brasil). The reagents used were of analytical grade, purchased from Vetec (São Paulo, Brazil).
For the antimicrobial evaluation, strains were used from the American Type Culture Collection (ATCC) (Rockville, MD, USA) Bacteria: Bacillus subtilis (ATCC 23858), Staphylococcus aureus (ATCC 6538P), Escherichia coli (ATCC 25922) and yeast Candida albicans (ATCC 10231).
Preparation of the Extract
Leaves of P. solmsianum C.D.C. var solmsianum (Piperaceae) were collected in May 2016 in Ponta Grossa, PR, Brazil, and identified by Dr. Elsie Franklin Guimarães. A voucher specimen was deposited at the Museu Botânico do Rio de Janeiro (RB 368597).
Small pieces were extracted with methanol (MeOH) at room temperature for 7 days. The MeOH extract was evaporated under reduced pressure with a rotary evaporator at 50 ºC to obtain the crude MeOH extract (CE).
Synthesis of nanocomposite containing silver
The CM (0.750 g) was dispersed in 50 mL of distilled water. The AgNO3 (0.085, 0.340, and 0.68 g) was solubilized separately, added to the mixture and, stirred for 20 min. Next, (0.1, 0.2 and 0.3 g) of CE, dissolved in 20 mL of methanol: ethanol (40:10) was added to the mixture. Concentrated ammonium hydroxide (0.06 mL) was also added, and the pH was adjusted to 11.0 with concentrated aqueous sodium hydroxide. The mixture was stirred for 120 min, protected from light, and heated to 80 ºC. Finally, the mixture was washed with distilled water and dried under vacuum for 24 h. The resulting composite, O-carboxymethylchitosan/γ-Fe2O3/Ag was reduced with P. solmsianum extract and was designated as CMAgPs.
Material characterization
The particle morphology was studied under transmission electron microscopy (TEM) using a transmission electron microscope (JEOL JEM-1011) operating at 100 kV at LCME/UFSC, Florianópolis, SC, Brazil. The samples were dispersed in isopropyl alcohol and placed in the ultrasound for 3 minutes. Then, 10 μL was added to a carbon-coated copper grid and air-dried. The images were analyzed using the ImageJ software program. The concentration of silver was determined by atomic absorption spectrophotometry (AAnalystTM 800, PerkinElmer). The calibration curve was prepared by serial dilutions of the stock solution 1000 mg L-1, using an assurance grade interference check standard (SPEX CertiPrep®).
The extract content of P. solmsianum present in the material was determined by the weight difference of the nanocomposites after Soxhlet extraction of the organic material, using methanol as solvent, for 24 h.
Antimicrobial assay
The minimum inhibitory concentrations (MIC) against bacteria and yeast strains were determined in 96-well microtiter plates by two-fold dilution of the nanocomposites using a standard broth microdilution of the antibacterial agents, following the guidelines of the Clinical and Laboratory Standards Institute (22,23), with minor modifications. Serial dilutions of the antibacterial material were performed in Mueller-Hinton (bacteria) and Sabouraud dextrose (yeast) broths, which were inoculated with a standardized number of organisms to obtain the final cell concentration equivalent to 5 x 104 cell mL-1 for bacteria and 0.5 x 103 to 5 x 103 cell mL-1 for yeast, and incubated at 35 ºC for 18 hours (bacteria) and 24-48 hours (yeast). The concentrations tested ranged from 1000 to 1.95 µg mL-1. Cell growth was determined by observing the turbidity of the culture. The lowest concentration of the materials at which no visual turbidity could be observed was considered to be the MIC of the antimicrobial materials.
Determination of time-kill kinetics
The time-kill kinetics were determined using the method proposed by CLSI (24) with adaptations. CMAgPs samples with the lowest MIC values for each microorganism were prepared and added to a test tube with 5 mL Mueller-Hinton (bacteria) and Sabouraud (yeast). Next, 10 µL inoculum at 0.5 x 108 cells mL-1, standardized by comparison with the McFarland scale, was added and the solution was homogenized. For the positive control, only the inoculum was added. 500 µL the solution with CMAgPs plus inoculum was serially dilutions in 0.86% sterile saline (10-1 to 10-8), and the same was done with the positive control (10-1 to 10-8). Next, 50 µL of each dilution was dispersed in Petri plates containing Mueller-Hinton agar and Sabouraud agar, spread with a Drigalski loop on the surface of the plate. This procedure was repeated after 2, 4, 8 and 24 hours. After each procedure, the plates were incubated at 35 ºC for 48 hours, and the colonies were counted considering the dilution factor for the calculations, and the results reported in colony forming unit per milliliter (CFU mL-1) and then extrapolated into Log10.
Exposure test of Artemia salina to CMAgPs
The tests were performed according to the protocol described and validated by Kos et al. (25). The dehydrated eggs of Artemia salina were hatched in saline medium (sea salt 38 g L-1) at pH 8.0. The eggs were dispersed in the medium (100 mg of eggs per 100 mL of medium) and incubated for 24 h under light and aeration.
The acute toxicity was determined by measuring the number of dead Artemia. The 24-hour-old nauplii were transferred to 24-well plates, with 10 nauplii in each, which were placed in contact with the materials and NPs (2 mL) at concentrations of 5, 10, 15, 25, 50, and 100 mg L-1. Ten replicates were used for each treatment. Saline medium was used as negative control, and a 60 mg L-1 solution of K2Cr2O7 was used as positive control. The plates were incubated in the dark at 24 °C. The number of dead nauplii was evaluated after 24 and 48 h, with immobile nauplii being considered dead. The test was considered valid if less than 10% of the control nauplii were immobile.
The mortality rate was calculated by following the equation:
Statistical Analysis
For each assay, the means were averaged from the replicates, and the error bars corresponded to the standard deviation of the mean. One-way analysis of variance (one-way ANOVA) was used, followed by Dunnett’s method and Tukey's test, to determine the statistical significance of each parameter among the treatments, with significant differences at p < 0.01 or 0.05. The statistical analyses were conducted using the software GraphPad Prism 5.0 (GraphPad Software).
Results and Discussion
Characterization of materials
The CM was previously prepared and characterized. It presented saturation magnetization, at room temperature, of 11.78 emu g-1 and the weight fraction of γ-Fe2O3 particles was estimated at ~18.6 wt % (1).
Silver and extract content
As the AgNO3 was mixed with the solution plant extract, the initial color changed from brown to black. The secondary metabolites present in the plants, such as phenols, terpenoids, alkaloids, carbohydrates, and proteins, which reduce Ag+ to Ag0, play an important role in the synthesis (26, 27). The relatively high levels of the eupomatenoid-3, eupomatenoid-5, conocarpan and orientin (18, 19), isoelemicin, syringaldehyde, 3,4,5-trimethoxy-benzoic acid, sitosterol, grandisin, rel-(7R,8R,70R,80R)-30,40-methylenedioxy-3,4,5,50-tetramethoxy-7,70-epoxylignan, rel-(7R,8R,70R,80R)-3,4,30,40-dimethylenedioxy-5,50-dimethoxy-7,70-epoxylignan(21); rel-(7R,8S,70S,80R)-3,4,5,30,40,50-hexamethoxy-7,70-epoxylignan,rel-(7R,8S,70S,8R0)-30,40-methylenedioxy-3,4,5,50-tetramethoxy-7,70-epoxylignan (28) may be act as reducing agents, or capping agents that provide stability to the silver nanoparticles.
The amount of silver present in the different materials is shown in Table 1. The results show that the amount of silver incorporated in the magnetic material increases as the amount of silver added to the reaction medium is increased. When the amount of silver added is small, the amount added to the extract has no influence on the amount of silver incorporated. The alkaline medium, the presence of free amino groups of O-carboxymethylchitosan, and a temperature of 80 ºC provide conditions for the formation of silver nanoparticles (1). The silver ions initially diffused into the CM, then they were uniformly anchored in the polymer network by many –NH2, –COOH, –OH, and –NHCOCH30 groups on the main chains of CM via coordination and electrostatic interactions and subsequent reduction to Ag0. The chitosan fraction present in O-carboxymethylchitosan (16%) may act as a reducing agent. In the reaction system, the nitrogen atom in the amines may lose one electron to generate its oxidized form, while the Ag+ ions may receive the electron to become Ag0 (29).
| Table 1. Amount of silver and Piper solmsianum extract present in eight different nanocomposites. |
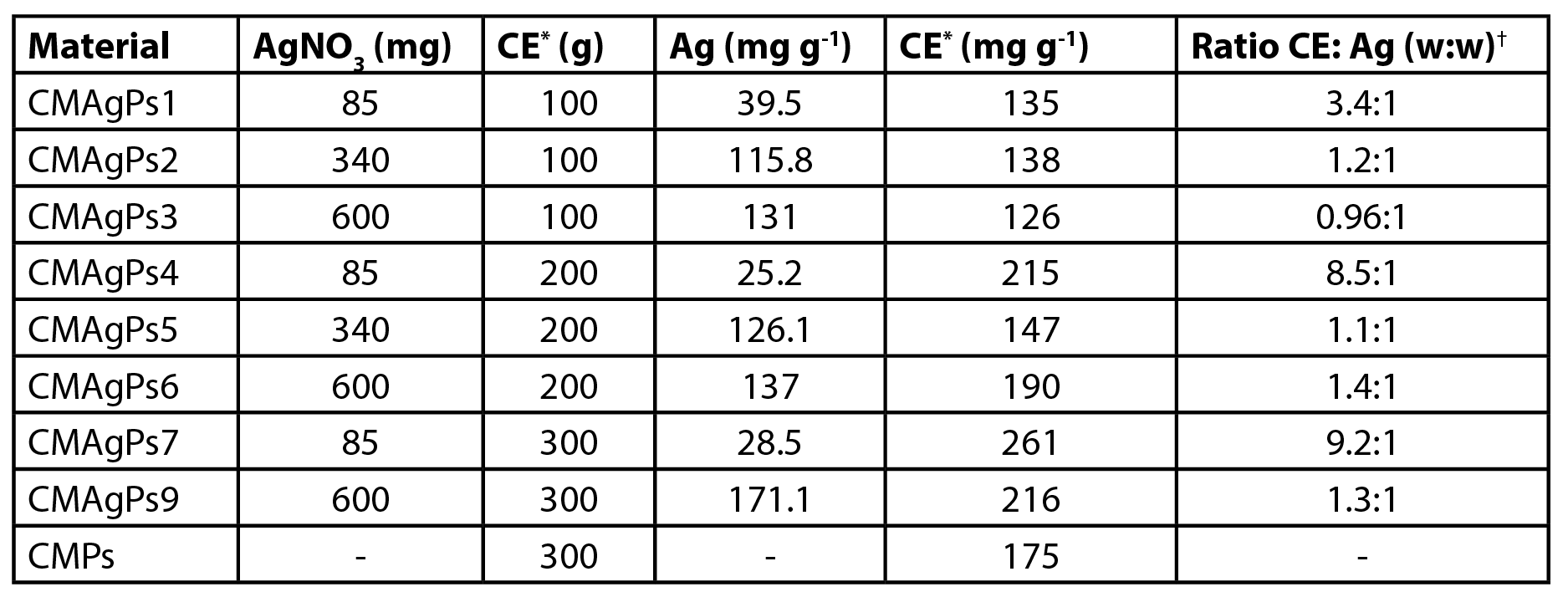 |
| *Crude Methanolic Extract; †Ratio CE: Ag (w:w) |
On the other hand, as the amount of silver added to the medium is increased, the amount of added extract has a direct influence on the amount of silver incorporated. In these cases, the presence of phytoconstituents with reducing properties is fundamental for the reduction of Ag+ to Ag0.
The amount of extract incorporated in the magnetic material varied between 12.6-26.1% (Table 1). When the materials were prepared with 0.1 g of extract, the total incorporation of the extract occurred. The resulting filtration solution was colorless (data not shown). When larger concentrations were used (0.2 and 0.3 g) the resulting solution had a greenish color, indicating the presence of soluble extract.
Structural characterization TEM
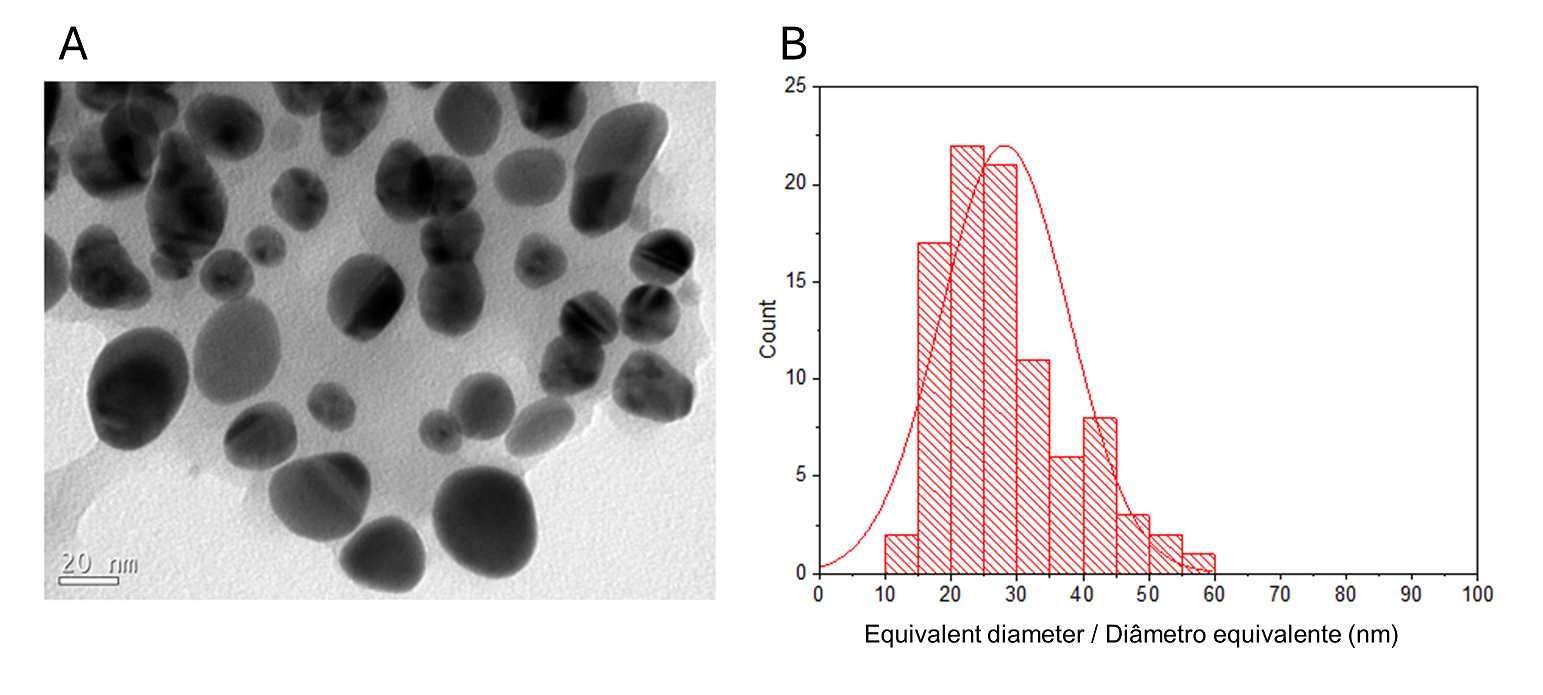
The detailed morphology and structural studies of O-carboxymethylchitosan/γ-Fe2O3 nanocomposite (CM), previously described, have shown that γ-Fe2O3 nanoparticles have an average diameter of 9.2 nm (1). The TEM image of the prepared CMAgPs with both Ag and γ-Fe2O3 particles in the O-carboxymethylchitosan/extract matrix is shown in Figure 1A.
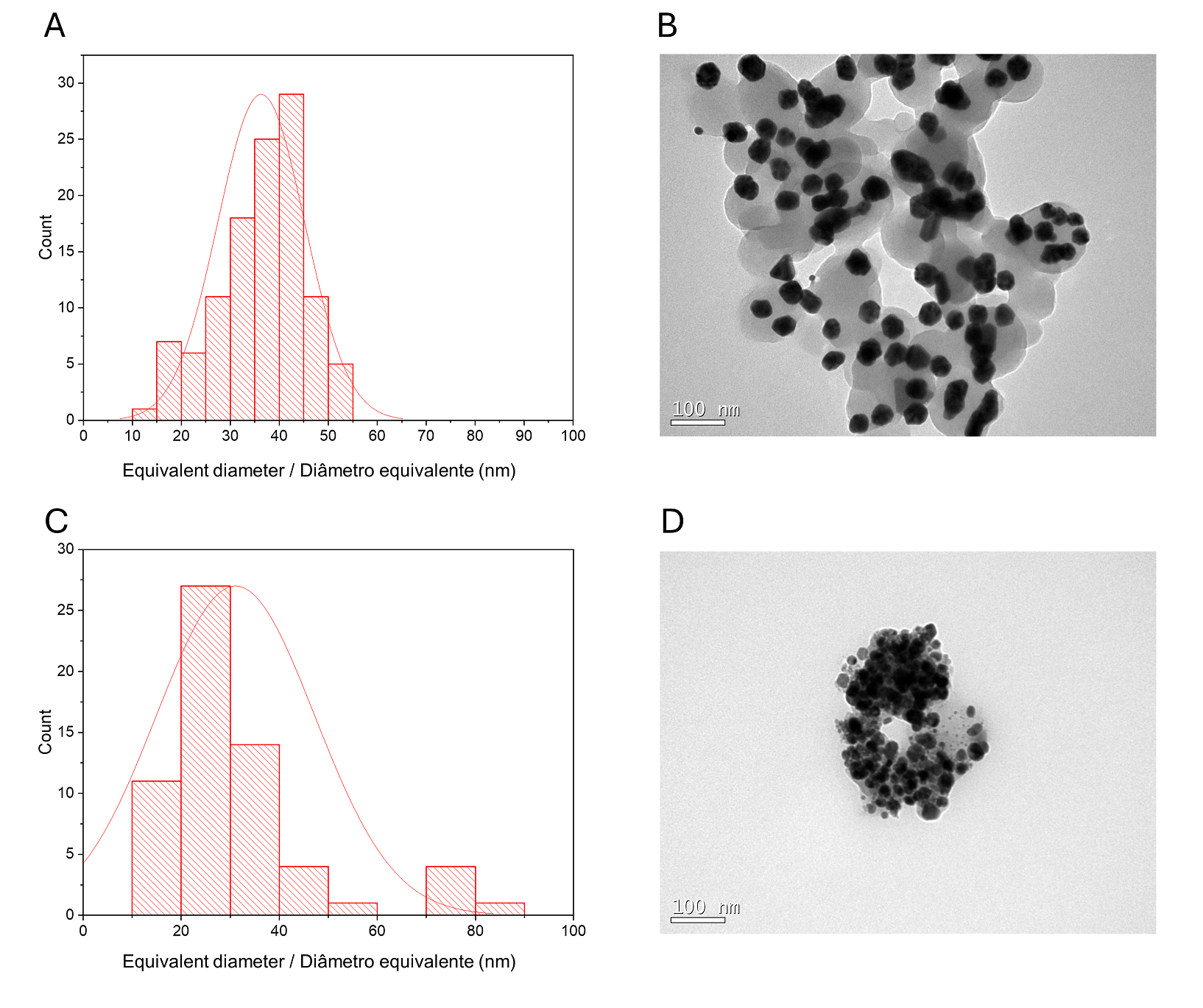
A histogram of particle sizes is shown in Figure 1B for CMAgPs9. It can be satisfactorily described by a lognormal distribution of particle diameters, giving an average grain size of 28.1 + 9.7 nm, and with broad size distribution (10-60 nm). The amount of extract present in the particles does not alter the particle size (Figure 2). The particle sizes found are larger than those reported when extracts from Eugenia umbelliflora, sucrose and NaBH4 (1) were used as a silver-reducing agent. Particle size depends on the amount of silver salt, the amount and type of capping, and the reducing agent (30, 31).
| Figure 1 - TEM image of CMAgPs9 nanocomposite (A) and histogram of particle size distribution (Ag and γ-Fe2O3) with a log-normal function (solid black line) obtained for γ-Fe2O3 particles in CMAgPs9 (B). |
 |
| Figure 2 - Histogram of particle size distribution (Ag and γ-Fe2O3) with a log-normal function (solid black line) obtained for γ-Fe2O3 particles in CMAgPs5 (A) and TEM image of CMAgPs5 nanocomposite (B), and in CMAgPs1 (C) and TEM image of CMAgPs1 nanocomposite (D). |
 |
Antimicrobial Activity
The antibacterial activity of the CMAgPs materials was quantitatively evaluated by determining the MIC against the pathogens. The results are shown in Table 2. As can be seen, all silver NP showed activity against all strains of organisms tested (bacteria and yeast), but the activity varied according to the silver and P. solmsianum CE concentrations in each sample.
| Table 2 - Minimum inhibitory concentration (MIC) of eight nanoparticles samples associated with different concentrations of Piper solmsianum extract. |
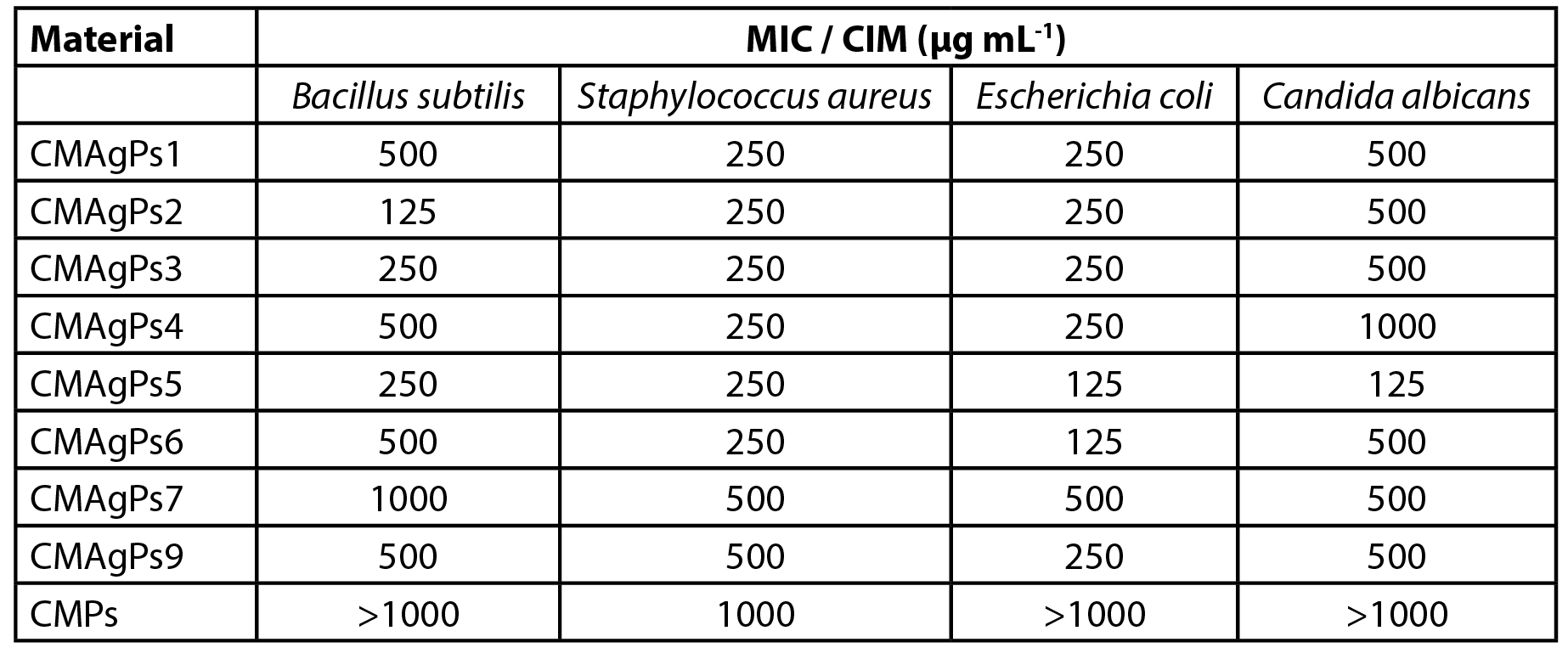 |
The minimum inhibitory concentration of the samples ranged from 125 to 1000 µg mL-1, and the most sensitive organism was E. coli. As can be seen, the increased amount of extract employed in the complexation of CM with AgNO3 did not potentiate the antimicrobial effect of the extract, but its addition acted as a silver reducing agent.
The concentration of P. solmsianum extract in the NP was 175 µg mL-1, which was sufficient for good antimicrobial activity, as it is reported that CE has an activity of between MIC 6-30 µg mL-1 for gram-positive bacteria and between MIC 20-30 µg mL-1 for fungi (18, 19). However, the silver-free NP showed poor activity against S. aureus, and was inactive for the other tested microorganisms.
The results of the antimicrobial activity presented by CMAgPs NP suggest that there is no relationship between the amount of silver and residual CE from P. solmsianum and antimicrobial activity, since it did not vary proportionally to the amount of silver and CE present.
There was no correlation between the MIC NP samples with the respective concentrations of CE and Ag. However, when the relationship between these two components in the NP samples is added to the analysis, it can be inferred that the NP with the best activity profiles (CMAgPs2, CMAgPs5 and CMAgPs6) were those in which the ratios of CE and Ag were between 1.1:1 and 1.4:1, suggesting that higher concentrations of CE may be disrupting the activity by covering the active sites of the Ag. The results also showed that low concentrations of Ag in PN are not sufficient for good antimicrobial activity.
Killing kinetics
Killing kinetics assays were performed based on the selection of NP, which presented the best inhibition profile (CMAgPs5) against E. coli and C. albicans. For E. coli, the analyses were based on the MIC (125 µg mL-1) and sub-inhibitory (62.5 µg mL-1) concentrations, and the results revealed that the NP (CMAgPs5) showed bactericidal action, where after the first hour, a marked reduction in viability was seen and after the fourth hour, cell viability for the concentration of 125 µg mL-1 was no longer seen. Also, as can be seen in Figure 3A, after 24 h, for both NP concentrations, there was a reduction in viability in the order of ≤ 6 Log10, relative to the control. Based on the result of the sub-inhibitory concentration, which still has a bactericidal effect, a concentration equivalent to ¼ of the MIC (15.62 µg mL-1) was also evaluated. At this concentration, the compound showed a reduction in the number of viable cells in the order of ≤ 3 Log10 until the eighth hour of incubation, compared to the control, and after this time there was an increase in the number of viable cells, showing that at this concentration, inhibition does occur, but the microorganism remains viable. A similar study was conducted by Haque et al. (32) with NPAg, which also obtained similar results against E. coli. Therefore, considering the concentration of the active principle against the microorganism, the inhibition time, and the unfeasibility, the results found for CMAgPs5 can be considered very promising.
For C. albicans, the analysis of killing kinetics data revealed a fungistatic effect of this NP against yeast, as it reduced the viable population (CFU mL-1) by ≤ 2 Log10, when compared to the control (Figure 3B). However, it was not able to completely kill this organism. Compounds with this type of action only inhibit microbial growth without necessarily rendering it inviable.
| Figure 3 - Time-kill curve of Escherichia coli (A) and Candida albicans (B) against the action of nanoparticle CMAgPs5. |
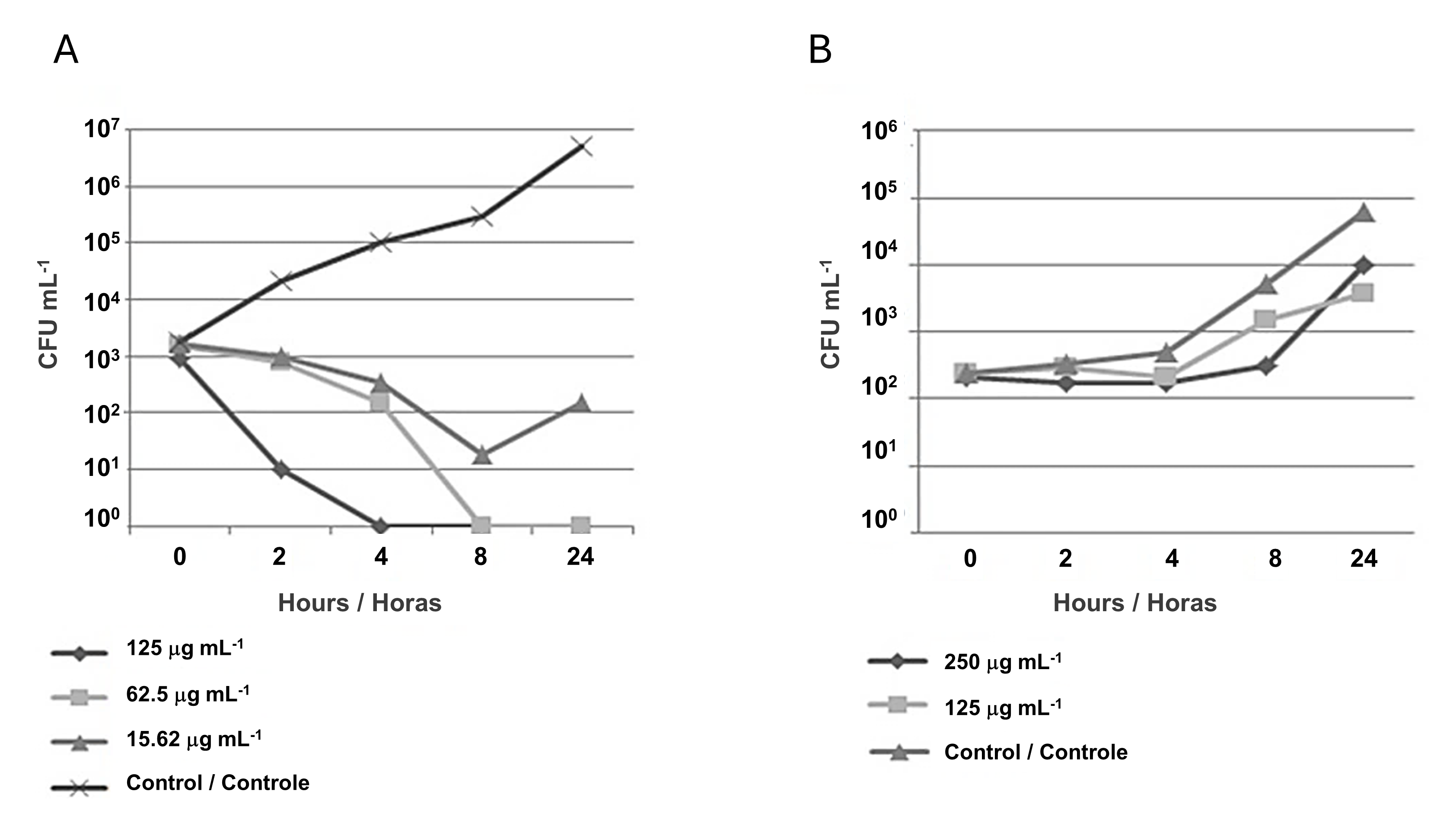 |
In general, the fungistatic effect is caused by some reversible metabolic damage in yeast and may grow back over time. In this experiment, for CMAgPs5, growth occurred after 4 h. Based on these results, the supra-inhibitory concentration was evaluated as twice the MIC (250 µg mL-1). It was observed that at this dose, the yeast kept the number of viable cells stable for 8 h without considerable growth, however, after this period, the viable number increased to control levels, and after this period, the number of viable increased, as did the control.
Toxicity associated with nanocomposite in Artemia salina nauplii
A. salina cytotoxicity is one of the most reliable methods of screening and detecting the cytotoxicity of a product. For this test, the compounds with the best antimicrobial activity results, as well as those that could cover from the lowest concentrations of Ag (CMPs and CMAgPs1) to the highest (CMAgPs9), passing through an intermediate concentration (CMAgPs5) and the same criterion for the concentration of CE. The respective results are shown in Table 3.
| Table 3 - Cytotoxicity of four selected nanocomposites and Piper solmsianum extract on Artemia salina. |
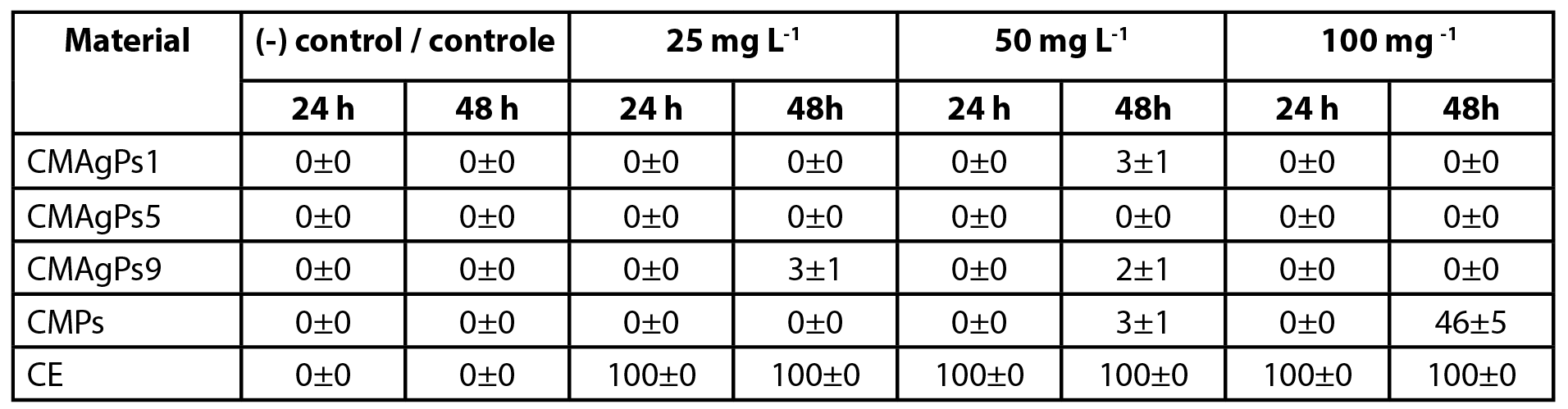 |
No nauplii died during the toxicity tests in the control group. After exposure for 24 h, no effect on survival of nauplii was observed for CMAgPs in the concentration range used. When the nauplii were in contact with CE, 100% death was observed within 24 h, over the entire concentration range. After exposure for 48 h, with the CMAgPs, 46% of the nauplii died at the concentration of 100 mg L-1 and the death of all nauplii was observed (p<0.001) for CE in the concentration range used.
The EC50 of P. solmsianum CE for nauplii has been reported to be 89.9 µg L-1 (18). This concentration is well below that presented in the present study, which was between 3.4 and 26.6 mg L-1. This result reveals that the incorporation of the extract in the magnetic material decreases its toxicity, which may be attributed to the decreased solubility of the extract incorporated into the magnetic material. The toxicity of CMPs and CE to A. salina nauplii may be linked to the cytotoxic activity of conocarpan, which has an IC50 of 2.9 µg L-1 (18), however, more studies are needed to elucidate what types of interactions are occurring so that toxicity is reduced. It is noteworthy that although the number of studies using the A. salina test has increased, due to several factors, as well as showing a good correlation of cytotoxicity (33), it does not replace other more specific and sensitive tests (34).
Conclusions
All the silver nanoparticles (NPs) obtained showed antimicrobial activity. The best results for antibacterial activity were obtained with CMAgPs2 against B. subtilis, CMAgPs5 and CMAgPs6 against E. coli (MIC 125 µg mL-1). The NPs from CMAgPs1 to CMAgPs6 showed good antibacterial activity against S. aureus (MIC of 250 µg mL-1), and CMAgPs5 also showed good activity on C. albicans (125 µg mL-1). The evaluation of killing kinetics revealed that CMAgPs5 showed bactericidal and fungistatic effects. Despite the recognized activity of CE, its potentiating effect of antimicrobial activity was not observed, but it has proven to be a potential reducing agent for green synthesis. In the toxicity test against Artemia salina, none of the NPs (CMAgPs) proved to be toxic.
Author contributions
The manuscript was produced through the contributions of all authors. F. Molinett, C.A. Rodrigues and A. Bella Cruz designed and performed the experiments and analysed the obtained results. The other authors conducted experiments for this study. F. Molinett wrote the manuscript and all co-authors provided constructive feedback on the manuscript.
Funding
This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001 and the Conselho Nacional de desenvolvimento Científico e Tecnológico- Brasil (CNPq). This work was partly conducted in a laboratory funded by POIG.02.02.00-00-025/09.
Acknowledgements
The authors would like to thank the LCME-UFSC for the technical support during the electron microscopy work.
Conflict of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Demarchi, C.A., Bella Cruz, A., Ślawska-Waniewska, A., Nedelko, N., Dłużewski, P., Kaleta, A., Trzciński, J., Magro, J.D., Scapinello, J. & Rodrigues, C.A. (2018). Synthesis of Ag@Fe2O3 nanocomposite based on o-carboxymethylchitosan with antimicrobial activity. International journal of biological macromolecules, 107(Pt A), 42–51. https://doi.org/10.1016/j.ijbiomac.2017.08.147
2. Arivizhivendhan, V., Mahesh, M., Boopathy, R., Karthikeyan, S., Mary, R.R. & Sekaran, G. (2018). Functioned silver nanoparticle loaded activated carbon for the recovery of bioactive molecule from bacterial fermenter for its bactericidal activity. Applied Surface Science, 427, 813–824. https://doi.org/10.1016/j.apsusc.2017.08.128
3. Shahriary, M., Veisi, H., Hekmati, M. & Hemmati, S. (2018). In situ green synthesis of Ag nanoparticles on herbal tea extract (Stachys lavandulifolia) modified magnetic iron oxide nanoparticles as antibacterial agent and their 4-nitrophenol catalytic reduction activity. Materials science & engineering. C, Materials for biological applications, 90, 57–66. https://doi.org/10.1016/j.msec.2018.04.044
4. Ahmad, S., Munir, S., Zeb, N., Ullah, A., Khan, B., Ali, J., Bilal, M., Omer, M., Alamzeb, M., Salman, S.M. & Ali, S. (2019). Green nanotechnology: a review on green synthesis of silver nanoparticles — an ecofriendly approach. International journal of nanomedicine, 14, 5087–5107. https://doi.org/10.2147/IJN.S200254
5. Abdelghany, T.M., Al-Rajhi, A.M.H., Al Abboud, M.A., Alawlaqi, M.M., Magdah, A.G., Helmy, E.A.M. & Mabrouk, A.S. (2018). Recent advances in green synthesis of silver nanoparticles and their applications: About future directions. A review. BioNanoScience 8, 5–16. https://doi.org/10.1007/s12668-017-0413-3
6. Majeed, A., Ullah, W., Anwar, A.W., Shuaib, A., Ilyas, U., Khalid, P., Mustafa, G., Junaid, M., Faheem, B. & Ali, S. (2018). Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: A review. Materials Technology: Advanced Performance Materials, 33, 313-320. https://doi.org/10.1080/10667857.2015.1108065
7. Shahid, M., Zhou, Y., Cheng, X-W., Zar, M.S., Chen, G. & Tang, R-C. (2018). Ferulic acid promoted in-situ generation of AgNPs@silk as functional colorants. Journal of Cleaner Production, 176, 736-744. https://doi.org/10.1016/j.jclepro.2017.12.171
8. Veisi, H., Azizi, S. & Mohammadi, M. (2018). Green synthesis of the silver nanoparticles mediated by Thymbra spicata extract and its application as a heterogeneous and recyclable nanocatalyst for catalytic reduction of a variety of dyes in water. Journal of Cleaner Production, 170, 1536-1543. https://doi.org/10.1016/j.jclepro.2017.09.265
9. Rafique, M., Sadaf, I., Tahir, M.B., Rafique, M.S., Nabi, G., Iqbal, T. & Sughr, K. (2019). Novel and facile synthesis of silver nanoparticles using Albizia procera leaf extract for dye degradation and antibacterial applications. Materials science & engineering. C, Materials for biological applications, 99, 1313-1324. https://doi.org/10.1016/j.msec.2019.02.059
10. WHO - World Health Organization. (2022). Drinking-water. Retrieved June 30, 2023, from https://www.who.int/news-room/fact-sheets/detail/drinking-water
11. Fan, M., Gong, L., Huang, Y., Wang, D. & Gong, Z. (2018). Facile preparation of silver nanoparticle decorated chitosan cryogels for point-of-use water disinfection. Science of The Total Environment 613-614, 1317-1323. https://doi.org/10.1016/j.scitotenv.2017.09.256
12. Sahbaz, D.A., Yakar, A. & Gündüz, U. (2019). Magnetic Fe3O4-chitosan micro- and nanoparticles for wastewater treatment. Particulate Science and Technology 37, 728-736. https://doi.org/1080/02726351.2018.1438544
13. Guimarães, E.F. & Carvalho-Silva, M. (2012). Piperaceae, In: Wanderley, M.G.L., Martins, S.E., Romanini, R.P., Melhem, T.S., Shepherd, G.J., Giulietii, A.M., Pirani, J.R., Kirizawa, M., Melo, M.M.R.F., Cordeiro, I., Kinoshita, L.S. (Eds.), Flora fanerogâmica do estado de São Paulo, v. 7, Instituto de Botânica, São Paulo, pp 263–320.
14. Reitz, R. (2003). Piperaceae, In: Flora Ilustrada Catarinense. Herbário Barbosa Rodrigues, Itajaí, Brazil.
15. Moreira, D.L., Kaplan, M.A. & Guimarães, E.F. (1995). Constituintes químicos de Piper solmsianumDC. (Piperaceae). Revista Brasileira de Farmácia, 76, 106-109.
16. Martins, R.C., Latorre, L.R., Sartorelli, P. & Kato, M.J. (2000). Phenylpropanoids and tetrahydrofuran lignans from Piper solmsianum. Phytochemistry 55, 843-846. https://doi.org/10.1016/S0031-9422(00)00295-8
17. Moreira, D.L., Souza, P.O., Kaplan, M.A., Pereira, N.A., Cardoso, G.L. & Guimarães, E.F. (2001). Effect of leaf essential oil from Piper solmsianum C.DC. in mice behaviour. Anais da Academia Brasileira de Ciências, 73, 33-37. http://dx.doi.org/10.1590/S0001-37652001000100004
18. Campos, M.P., Chechinel Filho. V., Silva, R.Z., Yunes, R.A., Zacchino, S., Juarez, S., Cruz, R.C.B. & Bella Cruz, A. (2005). Evaluation of antifungal activity of Piper solmsianum DC. var. solmsianum (Piperaceae). Biological and Pharmaceutical Bulletin, 28, 1527-1530. https://doi.org/10.1248/bpb.28.1527
19. Campos, M.P., Chechinel Filho, V., Silva, R.Z., Yunes, R.A., Monache, F.D. & Bella Cruz, A. (2007). Antibacterial activity of extract, fractions and four compounds extracted from Piper solmsianum DC. var. solmsianum (Piperaceae). Zeitschrift für Naturforschung C., 62, 173-178. https://doi.org/10.1515/znc-2007-3-404
20. Lopes, M.A., Ferracioli, K.R.C., Siqueira, V.L.D., de Lima Scodro, R.B., Cortez, D.A.G., da Silva, R.Z. & Cardoso, R.F. (2014). In vitro interaction of eupomatenoid-5 from Piper solmsianum C. DC. var. solmsianum and anti-tuberculosis drugs. The International Journal of Tuberculosis and Lung Disease, 18, 1513-1515. https://doi.org/10.5588/ijtld.14.0229
21. Martins, R.C., Lago, J.H., Albuquerque, S. & Kato, M.J. (2003). Trypanocidal tetrahydrofuran lignans from inflorescences of Piper solmsianum. Phytochemistry 64, 667-670. https://doi.org/10.1016/s0031-9422(03)00356-x
22. CLSI (2015). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically.Approved standard, 10th Document M07-A10, Clinical and Laboratory Standards Institute, Wayne, PA.
23. CLSI (2008). Method for broth dilution antifungal susceptibility testing of yeasts. Approved standard, 3th Document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
24. CLSI (1999). Methods for determining bactericidal activity of antimicrobial agents. Approved guideline, Document M26-A. Clinical Laboratory Standards Institute, Wayne, PA.
25. Kos, M., Kahru, A., Drobne, D., Singh, S., Kalčíková, G., Kühnel, D., Rohit, R., Gotvajn, A.Ž. & Jemec, A. (2016). A case study to optimise and validate the brine shrimp Artemia franciscana immobilisation assay with silver nanoparticles: The role of harmonisation, Environmental Pollution, 213, 173–183. https://doi.org/10.1016/j.envpol.2016.02.015
26. Sajjadi, G., Amini, J., Arani, A.S. & Nezammahalleh, H. (2018). Extracellular synthesis of silver nanoparticles using four fungal species isolated from lichens. IET Nanobiotechnology 12, 64-70. https://doi.org/1049/iet-nbt.2017.0170
27. Mazumder, J.A., Perwez, M., Noori, R. & Sardar, M. (2019). Development of sustainable and reusable silver nanoparticle-coated glass for the treatment of contaminated water. Environmental science and pollution research international, 26(22), 23070–23081. https://doi.org/10.1007/s11356-019-05647-4
28. Ramos, C.S., Linnert, H.V., Moraes, M.M., Amaral, J.H., Yamaguchi, L.F. & Kato, M.J. (2017). Configuration and stability of naturally occurring all-cis-tetrahydrofuran lignans from Piper solmsianum. RSC Advances, 7, 46932-46937. https://doi.org/10.1039/c7ra09262h
29. Biao, L., Tan, S., Wang, Y., Guo, X., Fu, Y., Xu, F., Zu, Y. & Liu, Z. (2017). Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Materials science & engineering. C, Materials for biological applications, 76, 73–80. https://doi.org/10.1016/j.msec.2017.02.154
30. Ebrahimzadeh, M.A., Derazkola, S.M. & Zazoul, M.A. (2019). Eco friendly green synthesis and characterization of novel Fe3O4/SiO2/ Cu2O–Ag nanocomposites using Crataegus pentagyna fruit extract for photocatalytic degradation of organic contaminants. Journal of Materials Science: Materials in Electronics, 30, 10994–11004. https://doi.org/10.1007/s10854-019-01440-8
31. Dizaji, A.N., Yilmaz, M. & Piskin, E. (2016). Silver or gold deposition onto magnetite nanoparticles by using plant extracts as reducing and stabilizing agents. Artificial cells, nanomedicine, and biotechnology, 44(4), 1109–1115. https://doi.org/10.3109/21691401.2015.1019672
32. Haque, M.A., Imamura, R., Brown, G.A., Krishnamurthi, V.R., Niyonshuti, I.I., Marcelle, T., Mathurin, L.E., Chen, J. & Wang, Y. (2017). An experiment-based model quantifying antimicrobial activity of silver nanoparticles on Escherichia coli. RSC Advances, 7, 56173-56182. https://doi.org/10.1039/C7RA10495B
33. Rajabi, S., Ramazani, A., Hamidi, M. & Naji, T. (2015). Artemia salina as a model organism in toxicity assessment of nanoparticles. Daru Journal of Pharmaceutical Sciences, 23(1), 20. https://doi.org/1186/s40199-015-0105-x.
34. Santos Filipe, M., Isca, V.M.S., Ntungwe, N.E., Princiotto, S., Díaz-Lanza, A.M. & Rijo, P. (2022). Lethality Bioassay using Artemia salina Journal of Visualized Experiments, 188, e64472. https://doi.org/10.3791/64472.
