![]()
Biopharmaceutical Sciences, Biomed Biopharm Res., 2021; 18(1):51-64
doi: 10.19277/bbr.18.1.253; download pdf verion [+] here
Systemic treatment of HER2-positive breast cancer patients with brain metastases: current status and exploratory case study in a Portuguese cohort
Paulo Luz 1,2 *, Elsa Campoa 1, Rita Gameiro 3, Marta Vaz 4, Isabel Fernandes 3, Joana Magalhães 1, Beatriz
Gosalbez 1, Sofia Braga 4, João Guilherme Costa 2, Ana Sofia Fernandes 2
1 Centro Hospitalar Universitário do Algarve, Medical Oncology;
2 CBIOS – Universidade Lusófona’s Research Center for Biosciences & Health Technologies, Lisboa, Portugal
3 Centro Hospitalar Barreiro-Montijo, Medical Oncology
4 Hospital Fernando Fonseca EPE, Medical Oncology
*corresponding author:
Abstract
Over the last years, the incidence of brain metastases in HER2 breast cancer patients has increased. Surgery and radiotherapy are the current standard local therapies. Nevertheless, it is unclear which and when systemic treatment should be applied in addition to local treatment. This work aims to present an updated review of current systemic treatment options for patients with HER2+ metastatic breast cancer with brain metastases and to present a case study of clinical cases that occurred in a Portuguese population.
The methodology of this work included a literature search in PubMed for the impact of HER2-targeting agents, such as pertuzumab, trastuzumab emtansine (T-DM1), lapatinib, neratinib, trastuzumab deruxtecan, and tucatinib in the treatment of patients with HER2+ breast cancer with brain metastases. Then, a cohort of Portuguese patients with HER2+ breast cancer (n=44) was analyzed. In this exploratory study, considering a follow-up of 23.9 months, three patients (6.8%) developed brain metastases despite having shown a complete pathological response. The role of systemic treatment for patients with HER2 breast cancer with brain metastases has rapidly evolved following recent successes in phase II and III clinical trials. The biggest challenge is how to integrate systemic and local treatment in the management of these patients. health.
Keywords: Brain metastases, breast cancer, HER2, trastuzumab deruxtecan, tucatinib
Received: 15/11/2020; Accepted: 19/03/2021
Introduction
Up to 15% of all patients with metastatic breast cancer (BC) will develop brain metastases during the course of their disease. The molecular subtypes with a higher risk of developing brain metastases are triple-negative breast cancer and HER2+ tumors (1). The incidence of brain metastases has been rising over the last years, commonly attributed to the excellent systemic control that increases the overall survival (OS) of BC patients.
According to several studies, the presence of brain metastases in HER2+ BC occurs in about 10-14% after diagnosis of early-stage disease and 28-41% in metastatic settings (1, 2).
In terms of survival, a retrospective study carried out at Institut Jules Bordet, Belgium, with 483 patients, 72 of which had brain metastasis, revealed an overall survival (OS) of 20.8 months, compared with 46.7 months of OS in the group of patients with metastatic disease without disease in the brain (1). Over the years, therapeutic developments, namely anti-HER2 therapies, have changed the paradigm associated with this tumor subtype and its OS. With these developments, there was an increase in OS from 2-16 months to 14-24 months (3).
For treating brain metastasis, surgical intervention is preferable whenever possible, namely in single lesions with small size and easy surgical access (3), although radiotherapy is also an option. In randomized trials that included patients with lung and breast cancer, the median survival in patients treated with whole-brain radiotherapy (WBRT) ranges from four to six months, at the cost of some relevant toxicity, namely cognitive impairment (4-6). The development of stereotactic techniques has also significantly improved the quality of life of these patients, associated with less neurological toxicity (1-3). With the advent of multiple therapies, the paradigm has changed. New systemic approaches have been shown to increase patient survival and the maintenance of functionality in daily activities (1,3).
This article aims to review the different systemic therapies currently available for the treatment of HER2+ BC patients with brain metastases (BM). Moreover, a cohort of Portuguese patients with HER2+ breast cancer was analyzed and a case study of the patients that developed BM is presented.
Materials and Methods
Literature review
A literature search in the PubMed database, using the keywords “HER2 breast cancer” and “brain metastases” was conducted. Articles published in English between January 2006 and June 2020 were selected. In this narrative review, original articles were selected based on their clinical and scientific relevance.
The objective was to describe the impact of the HER2- targeting agents pertuzumab, trastuzumab emtansine (T-DM1), lapatinib, neratinib, trastuzumab deruxtecan and, tucatinib on the treatment of patients with HER2+ breast cancer and BM.
Observational study
Patients with HER2+ BC who were diagnosed and treated at Centro Hospital Universitário do Algarve and Centro Hospitalar Barreiro-Montijo between January 2018 and December 2019, were included in this study.
Additional inclusion criteria were patients with HER2+ breast carcinoma, submitted to neoadjuvant therapy with double anti-HER2 block with trastuzumab and pertuzumab, followed by surgery. We excluded patients with stage IV disease at diagnosis and patients who progressed during neoadjuvant therapy.
The following parameters were analyzed by reviewing the electronic medical records of the patients and according to oncology and pathology services protocols: initial stage, lymph nodes status, expression of hormone receptors, Ki-67, type of surgery, type of adjuvant therapy, distant recurrence, and pathological response.
The cases where brain metastases were detected during this preliminary follow-up were characterized.
Descriptive statistics were used whenever appropriate.
Results and Discussion
According to our literature research, the following HER2-targeting agents may be used in the treatment of patients with HER2+ breast cancer and brain metastases: pertuzumab, trastuzumab emtansine (TDM1), lapatinib, neratinib, trastuzumab deruxtecan and, tucatinib. In the following sub-sections, the clinical use of these drugs for this purpose is reviewed.
Monoclonal antibodies
Pertuzumab
Pertuzumab is a monoclonal antibody that prevents heterodimerization of the HER2 receptor with other receptors in the EGFR family (8). The study that led to its approval in first-line treatment for patients with metastatic HER2+ BC (CLEOPATRA) excluded patients with brain metastases. However, in the followup of these patients, it was found that therapy with trastuzumab, pertuzumab, and docetaxel did not reduce the incidence of brain metastasis but delayed its onset (11.9 months in the placebo group versus (vs.) 15
months in the pertuzumab-treated group, HR = 0.58, 95% CI 0.39-0.85, p =0.0049) (7). Final results of the CLEOPATRA study showed 8-year overall survival rates of 37% (95% CI 31–42) in the pertuzumab group and 23% (95% CI 19–28) in the placebo group (8).
In a real-life study, known as RePER, on the use of double anti-HER2 blockade first-line treatment, 21 of the 264 patients presented brain metastases at baseline.
The rate of 2-year OS was 77.7% in those patients and 83.9% in patients without brain metastases (9).
Antibody-drug conjugates
Trastuzumab-emtansine
Trastuzumab-emtansine (T-DM1) is a conjugated monoclonal antibody bound with tubulin inhibitor maytansine (10). The EMILIA trial evaluated the efficacy of T-DM1 vs. lapatinib and capecitabine. This study included 95 patients with brain metastasis. In this subset of patients, T-DM1 has shown an increase in OS (hazard ratio 0.38, p = 0.008, mean 26.8 vs. 12.9 months) (10). However, this data should be interpreted with caution since no increase in progression-free survival (PFS) was observed and, therefore, this difference in OS may be related to subsequent therapies and extracranial disease control (10).
In the KAMILLA study, the largest observational study of T-DM1-treated patients to date, patients with brain metastasis (n = 398) showed similar results to those without metastasis to this organ (PFS of about 7 months and OS of 27 months) (11). It is necessary to consider that this study excluded patients with neurological symptoms caused by brain metastases or with brainlimited metastasis. Special consideration needs to be given to the risk for radionecrosis associated with this agent (12).
Trastuzumab deruxtecan
Trastuzumab deruxtecan is an antibody-drug conjugate composed of an anti-HER2 antibody, a cleavable tetrapeptide-based linker, and a cytotoxic topoisomerase I inhibitor (13).
The phase II DESTINY-Breast01 study (13) examined the efficacy of trastuzumab deruxtecan in patients with previously treated HER2+ BC who had received previous treatment with T-DM1. Data showed that in 184 patients who had undergone a median of six previous lines of treatment, the confirmed objective response rate (ORR) with trastuzumab deruxtecan was 60.3% and the median duration of response (DOR) was 14.8 months. The median PFS was 16.4 months (95% CI, 12.7 to not reached) among all patients and 18.1 months (95% CI, 6.7 to 18.1) among the 24 enrolled patients with treated and asymptomatic brain disease.
Estimated OS at 6 months was 93.9% (95% CI, 89.3 to 96.6) and 86.2% (95% CI, 79.8 to 90.7) at 12 months; median OS was not reached at the time of the publication. The most frequent grade 3 or 4 side effects were neutropenia (20.7%), anaemia (8.7%), and nausea (7.6%). Adverse events that led to discontinuation included pneumonitis (in 11 patients) and interstitial lung disease (in 5 patients) (13).
Small molecule tyrosine kinase inhibitors
Lapatinib
Lapatinib is a reversible tyrosine kinase inhibitor of the EGFR (HER1) and HER2 receptors (14). In the LANDSCAPE clinical trial, Bachelot et al. investigated the role of the combination of lapatinib with capecitabine in 45 patients without previous WBRT treatment. The results showed a partial response to the lesions in 65.9% of cases and achieved an improvement of neurological symptoms in 58% of cases. The average time until progression to the brain was 5.5 months (14).
Lapatinib was also tested in combination with trastuzumab. Lin et al. (15) reported an objective brain response rate of 79% and a median OS of 19 months in a prospective study with 28 patients. Furthermore, Bartsch et al. (16) showed a significant prolongation of OS in the group treated with lapatinib and trastuzumab (p = 0.002) in a retrospective study (25 patients).
The role of lapatinib to prevent brain metastases was tested in the CEREBEL trial, where patients were randomly assigned to receive lapatinib-capecitabine or trastuzumab-capecitabine (17). Brain metastases as the first site of relapse occurred in 3% (8 of 251 patients) of the patients treated with lapatinib and 5% (12 of 250 patients) in the trastuzumab group (95% CI, -2% to 5%; p=0.360). The trial was inconclusive for the primary endpoint.
Neratinib
Neratinib is an HER2 receptor tyrosine kinase inhibitor.
It irreversibly inhibits the EGFR receptor (HER1), HER2 and HER4 and demonstrated activity in the brain in two clinical trials (18,19).
In the TBCRC 022 study, a phase 2 trial, 49 HER2+ BC patients with brain metastases were treated with neratinib and capecitabine. Twelve of the patients had previously been treated with lapatinib (18). The ORR was 33% and 49% in lapatinib-treated and lapatinibnaive patients, respectively. PFS in pre-treated and lapatinib-naive patients was 3.1 and 5.5 months, respectively, and OS was 15.1 and 13.3 months (18).
The NEfERT-T phase 3 trial (20) randomized 479 women to receive neratinib or trastuzumab combined with paclitaxel as first-line therapy for recurrent or metastatic HER2 disease (3). There was no significant difference in PFS, however, the neratinib group did have a lower incidence of brain recurrence (RR 0.48, 95%
CI, 0.29-0.79; p = 0.002), as well as a longer time to recurrence at this level (HR 0.45, 95% CI, 0.26-0.78; p=0.004). Grade 3 diarrhea was also a very common and relevant toxicity effect in the neratinib group (30.4% vs. 3.8%).
In the NALA study, (19) a phase III clinical trial, 621 patients (including 101 patients with asymptomatic or stable brain metastases) were randomized into two groups. One group was treated with neratinib and capecitabine and the other group with lapatinib and capecitabine. The risk of progression or death was reduced by 24% with neratinib and capecitabine (HR = 0.76; 95% CI 0.63–0.93; p = 0.006). The PFS at 6 and 12 months was 47.2% vs 37.8% and 28.8% vs 14.8% for the neratinib + capecitabine and lapatinib + capecitabine groups, respectively. OS was also superior in the neratinib + capecitabine group, although not statistically significant (HR = 0.88; 95% CI 0.72–
1.07; p = 0.2086). The incidence of intervention on symptomatic brain metastases was lower in the neratinib group (23%) compared to the lapatinib group (29%) (p= 0.043). Again, diarrhea was the most frequent toxicity effect related to neratinib in all clinical trials, being grade 3 in about 30% of the patients.
Tucatinib
Tucatinib is a tyrosine kinase inhibitor with a high affinity for the kinase domain of the HER2 receptor and with minimal inhibition of the EGFR receptor, which improves the toxicity profile (21).
In the HER2CLIMB trial, 410 patients with progressing metastatic HER2+ BC after treatment with trastuzumab, pertuzumab, and TDM-1 were randomized to receive treatment with trastuzumab and capecitabine with or without tucatinib (21). About 48% of patients had brain metastases that did not require local approach therapies.
PFS was superior with tucatinib vs. placebo (7.8 vs. 5.6 months; hazard ratio, 0.54), as well as OS (21.9 vs. 17.4 months; HR, 0.66) and ORR (41% vs 23%). Among the patients with brain metastases, the estimated PFS at 1 year was 24.9% (95% CI, 16.5 to 34.3) in the tucatinib-combination group and 0% in the placebo combination group. The median duration of PFS was 7.6 months (95% CI, 6.2 to 9.5) and 5.4 months (95% CI, 4.1 to 5.7), respectively. In the tucatinib group, the most frequent adverse events included diarrhea, palmar–plantar erythrodysesthesia syndrome, fatigue, and nausea.
In a phase 1b trial (22) the combination of tucatinib and T-DM1 showed an objective response rate of 36% in 14 patients with brain metastases (2 patients had a complete response, 3 had a partial response, 7 had stable disease, and 2 were non-evaluable). This combination is under evaluation, in a phase 3 trial (NCT03975647).
Observational study
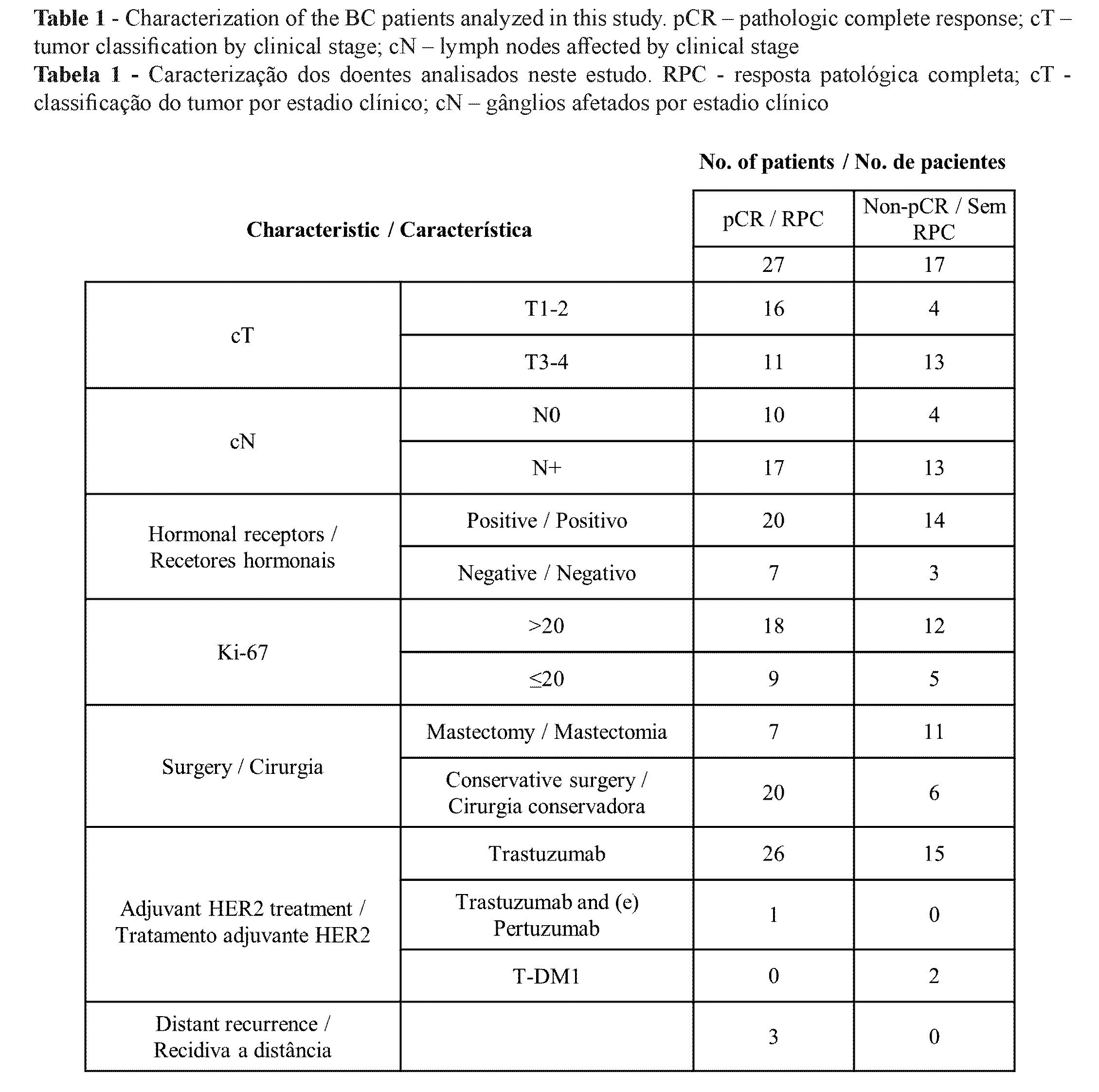
Our sample included 44 Portuguese patients with HER+ breast carcinoma, submitted to neoadjuvant therapy with double anti-HER2 block with trastuzumab and pertuzumab, followed by surgery. The average age of the patients was 55 years. After neoadjuvant therapy, 18 patients underwent mastectomy and 26 underwent conservative surgery. The rate of complete pathological response in all patients was 61.4%. The characterization of the patients according to their pathologic response is shown in Table 1. In patients who expressed hormone receptors (n=34), the response rate was 58.8%, while in HER2-enriched patients (n=10) it was 70%. Regarding the influence of the tumor size, the complete response rate was 80% in patients with T1 or T2 tumors and 45.8% in patients with T3 or T4. The rate of complete pathological response was 71.4% in patients without nodal involvement and 56.7% in patients with affected nodes.
Case study of HER2+ BC patients with brain metastases
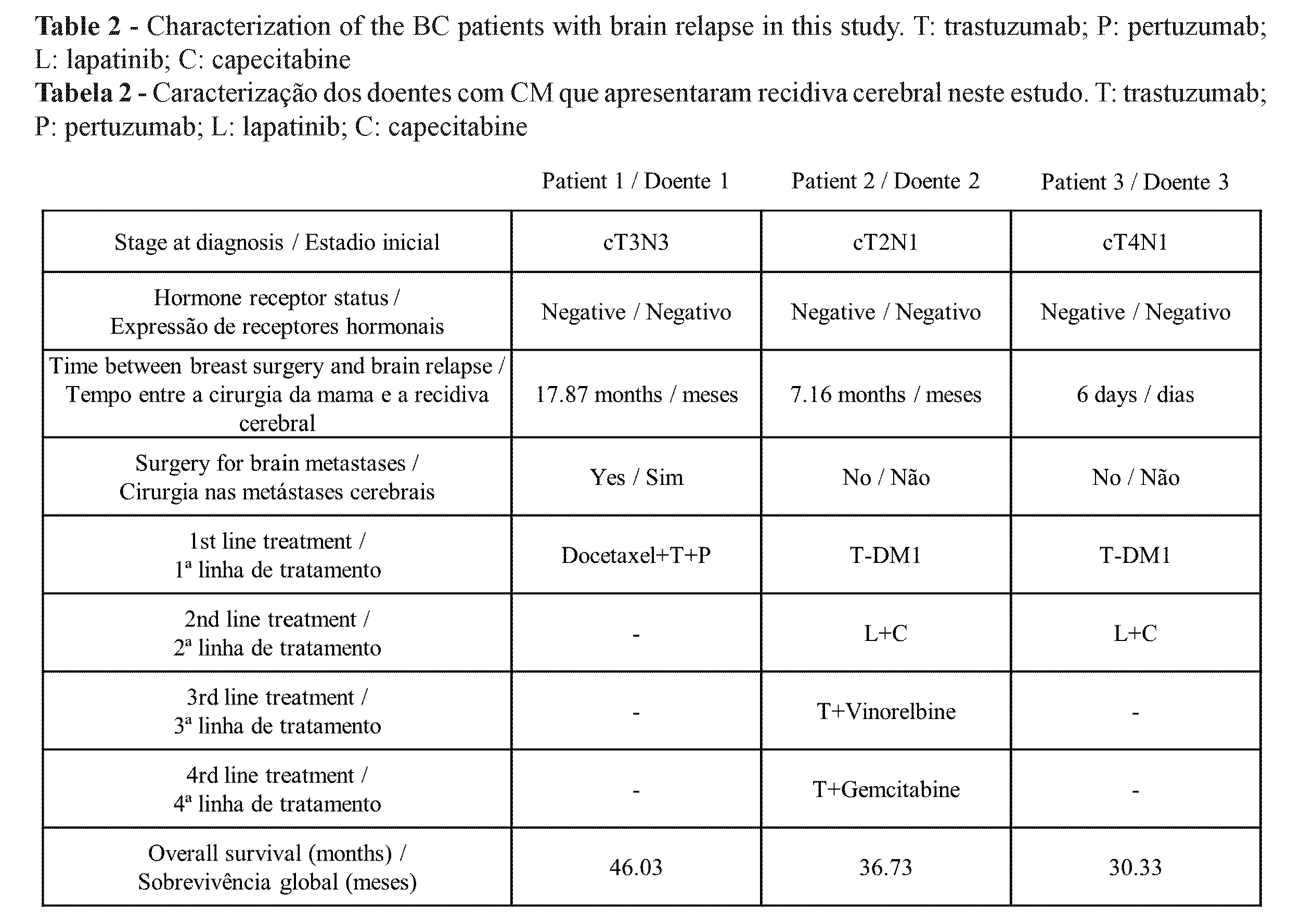
Of the 44 patients treated with neoadjuvant trastuzumab and pertuzumab with a mean follow-up of 23.9 months, three had a relapse, all at the brain level as the first site of metastasis. Interestingly, these three patients had shown a complete pathological response. The characterization of these patients is shown in Table 2. In Patient 1, relapse occurred 17.87 months after breast surgery.
The brain lesion was resected, but, unfortunately, brain progression occurred one month after surgery. She received WBRT and then started systemic treatment.
In Patients 2 and 3, brain relapse occurred soon after breast surgery. The three patients received WBRT and then started systemic treatment. To date, Patient 1 is under first-line treatment with trastuzumab and pertuzumab with stable disease and with no extra-brain disease. Patients 2 and 3 have died.
Only a few reports have examined risk factors for the development of brain metastases as the first site of metastatic disease in HER2+ breast cancer patients who received neoadjuvant treatment. In a cohort of 130 patients treated with neoadjuvant chemotherapy with trastuzumab and pertuzumab, the rate of BM as the first presentation of distant disease was 3.8% (3/77) and 3.7% (2/53) in patients with pCR and non-pCR, respectively (23). However, the median time to development of BM was longer in patients with pCR (35 vs 11.7 months).
In our cohort, brain relapse occurred in 1.1% (3/27) of patients with pCR. These three patients presented large tumors with nodal involvement, which is a risk factor for brain relapse (1). Overall survival of these patients was in accordance with previous studies (3).
In the Katherine study, the incidence of BM at the follow-up of 3 years was 5.9% in patients with residual disease who received TDM1 and 4.3% in patients who received trastuzumab (24). Our very small number of patients treated with adjuvant T-DM1 does not allow us to make conclusions about the effect of this treatment, but at the time of assessment, no patients had relapsed.
Also, the short period between surgery and brain recurrence in 2 out of 3 patients raises questions about the possibility of preexisting metastases at the time of surgery and whether there is a rationale for brain metastases screening in these patients. At this point, no recommendations can be made about this issue. Some current clinical trials are studying the role of brain MRIs in screening for brain metastases before the onset of symptoms and whether this will affect the overall prognosis and quality of life of patients (NCT03617341, NCT03881605, NCT04030507).
Conclusion
With the development of new molecules with activity in the brain, the biggest challenge is how to integrate surgery and radiotherapy in clinical treatment. As patient survival increases, the consequences of longterm treatments will be observed, namely the cognitive deterioration resulting from radiotherapy. Multiple prognostic models exist to help oncologists make decisions about patients who develop brain metastasis.
One of the most used prognostic models is the Modified Breast Graded Prognostic Assessment (GPA), which confirmed that tumor receptor status, age, performance status, and number of BMs can predict OS (25).
Surgery will always play an important role, especially in the case of solitary brain lesions. It has the advantages of reducing tumor burden and allowing a revaluation of the immunochemistry markers. Discordance in hormone receptors and HER2 status between the. primary tumor and the metastatic lesion is well known.
A large range of receptor discordance with ER, PR, and HER2 (6–40%, 21–41%, and 1–43%, respectively) was described in a previous review (26). Radiosurgery is also an option for some unresectable brain metastases.
Current guidelines recommend that for patients with stable systemic disease at the time of BM diagnosis, the same systemic therapy should be continued, and BM should be treated with local therapy (surgery and/or radiotherapy). For patients with progressive systemic disease at the time of BM diagnosis, clinicians should
use the algorithms for the treatment of HER+ metastatic BC (27). These guidelines may change in the very near future, and it may be wise for oncologists to change systemic therapies when brain recurrence occurs even with stable systemic disease. Trastuzumab deruxtecan or tucatinib can be considered in these cases.
Asymptomatic or lightly symptomatic patients with largely disseminated BM at diagnosis are normally referred for treatment with WBRT as a local therapy, however, this may not be the best choice. A change to systemic therapy may be more sensible, even with stable systemic disease, delaying radiotherapy until there is disease progression or there are symptoms related to metastasis. We will also see what role new targeted therapies like trastuzumab, deruxtecan, and tucatinib will play in early disease stages, when they might prevent or delay the onset of metastasis to the brain.
In this study, the neoadjuvant therapy with double anti-HER2 block using trastuzumab and pertuzumab led to higher rates of complete pathological response in patients with lower clinical stage and without nodal involvement. With a preliminary mean follow-up of 23.9 months, three patients (of 44 studied) had a relapse, and the brain was the first site of metastasis in all cases.
The brain may be ceasing to be the sanctuary that we thought it was. The appearance of new molecules with activities in the brain in HER2+ BC inspires new hope for the treatment of these patients. Further investigation is also needed in order to prevent HER2+ BC brain metastases.
Author Contributions Statement
PL: Investigation, Methodology, Writing – Original Draft Preparation; EC: Investigation, Methodology, Writing – Original Draft Preparation; RG: Writing – Original Draft Preparation; MV: Investigation, Methodology, Writing – Original Draft Preparation; IF: Writing – Original Draft Preparation ; JM: Validation; BG: Validation ; SB: Validation ; JGC: Supervision, Writing - Review & Editing; ASF: Supervision, Writing – Review & Editing
Funding
The authors received no financial support for the research, authorship, or publication of this article.
Conflict of Interests
The authors report no conflicts of interest.
References
- Maurer, C., Tulpin, L., Moreau, M., Dumitrescu, C., de Azambuja, E., Paesmans, M., Nogaret, J. M., Piccart, M. J., & Awada, A. (2018).
Risk factors for the development of brain metastases in patients with HER2-positive breast cancer. ESMO open, 3(6), e000440. https://
doi.org/10.1136/esmoopen-2018-000440 - Kennecke, H., Yerushalmi, R., Woods, R., Cheang, M. C., Voduc, D., Speers, C. H., Nielsen, T. O., & Gelmon, K. (2010). Metastatic
behavior of breast cancer subtypes. Journal of Clinical Oncology :Official Journal of the American Society of Clinical Oncology,
28(20), 3271–3277. https://doi.org/10.1200/JCO.2009.25.9820 - McKee, M. J., Keith, K., Deal, A. M., Garrett, A. L., Wheless, A. A., Green, R. L., Benbow, J. M., Dees, E. C., Carey, L. A., Ewend, M.
G., Anders, C. K., & Zagar, T. M. (2016). A Multidisciplinary Breast Cancer Brain Metastases Clinic: The University of North Carolina
Experience. The Oncologist, 21(1), 16–20. https://doi.org/10.1634/theoncologist.2015-0328 - Mehta, M. P., Rodrigus, P., Terhaard, C. H., Rao, A., Suh, J., Roa, W., Souhami, L., Bezjak, A., Leibenhaut, M., Komaki, R., Schultz,
C., Timmerman, R., Curran, W., Smith, J., Phan, S. C., Miller, R. A., & Renschler, M. F. (2003). Survival and neurologic outcomes in a
randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. Journal of Clinical Oncology:
Official Journal of the American Society of Clinical Oncology, 21(13), 2529–2536. https://doi.org/10.1200/JCO.2003.12.122. - Suh, J. H., Stea, B., Nabid, A., Kresl, J. J., Fortin, A., Mercier, J. P., Senzer, N., Chang, E. L., Boyd, A. P., Cagnoni, P. J., & Shaw, E.
(2006). Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. Journal of Clinical
Oncology: Official Journal of the American Society of Clinical Oncology, 24(1), 106–114. https://doi.org/10.1200/JCO.2004.00.1768 - Borgelt, B., Gelber, R., Kramer, S., Brady, L. W., Chang, C. H., Davis, L. W., Perez, C. A., & Hendrickson, F. R. (1980). The palliation
of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. International Journal of
Radiation Oncology, Biology, Physics, 6(1), 1–9. https://doi.org/10.1016/0360-3016(80)90195-9 - Swain, S. M., Baselga, J., Miles, D., Im, Y-H., Quah, C., Lee, L. F., & Cortés, J. (2014). Incidence of central nervous system metastases in
patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized
phase III study CLEOPATRA. Journal of Clinical Oncology: Official Journal of the American Society of Clinical
Oncology, 25(6), 1116–1121. https://doi.org/10.1093/annonc/mdu133 - Swain, S.M, Miles D., Kim, S-B, Im, Y-H., Im, S-A. Semiglazov, V., Ciruelos, E., Schneeweiss, A. Monturus, E., Clark, E.,
Knott, A. Restuccia, E., Benyunes, M., Cortes, J. (2019). End-of-study analysis from the phase III, randomized, double-blind,
placebo-controlled CLEOPATRA study of first-line pertuzumab, trastuzumab, and docetaxel in patients with
HER2-positive metastatic breast cancer. ASCO Annual Meeting. Abstract 1020. Presented June 2, 2019. Journal of Clinical
Oncology: Official Journal of the American Society of Clinical Oncology, 37 (suppl; abstr 1020). https://
doi.org/10.1200/JCO.2019.37.15_suppl.1020 - Gamucci, T., Pizzuti, L., Natoli, C., Mentuccia, L., Sperduti, I., Barba, M., Sergi, D., Iezzi, L., Maugeri-Saccà, M., Vaccaro, A.,
Magnolfi, E., Gelibter, A., Barchiesi, G., Magri, V., D'Onofrio, L., Cassano, A., Rossi, E., Botticelli, A., Moscetti, L., Omarini, C., …
Vici, P. (2019). A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for
advanced HER2 positive breast cancer patients. RePer Study. Cancer Biology & Therapy, 20(2), 192–200. https://doi.org/10.1080/153
84047.2018.1523095 - Krop, I. E., Lin, N. U., Blackwell, K., Guardino, E., Huober, J., Lu, M., Miles, D., Samant, M., Welslau, M., & Diéras, V. (2015).
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central
nervous system metastases: a retrospective, exploratory analysis in EMILIA. Annals of Oncology: Official Journal of the European
Society for Medical Oncology, 26(1), 113–119. https://doi.org/10.1093/annonc/mdu486 - Montemurro F, Ellis P, Delaloge S. (2017). Safety and efficacy of trastuzumab emtansine (T-DM1) in 399 patients with central nervous
system metastases: Exploratory subgroup analysis from the KAMILLA study. Abstract No. P1–12–10, 2016 San Antonio Breast
Cancer Symposium. Cancer Research 77(4 Supplement) P1-12-10. https://doi.org/10.1158/1538-7445.SABCS16-P1-12-10 - Stumpf, P. K., Cittelly, D. M., Robin, T. P., Carlson, J. A., Stuhr, K. A., Contreras-Zarate, M. J., Lai, S., Ormond, D. R., Rusthoven,
C. G., Gaspar, L. E., Rabinovitch, R., Kavanagh, B. D., Liu, A., Diamond, J. R., Kabos, P., & Fisher, C. M. (2019). Combination of
Trastuzumab Emtansine and Stereotactic Radiosurgery Results in High Rates of Clinically Significant Radionecrosis and
Dysregulation of Aquaporin-4. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research,
25(13), 3946–3953. https://doi.org/10.1158/1078-0432.CCR-18-2851. - Modi, S., Saura, C., Yamashita, T., Park, Y. H., Kim, S. B., Tamura, K., Andre, F., Iwata, H., Ito, Y., Tsurutani, J., Sohn, J., Denduluri, N.,
Perrin, C., Aogi, K., Tokunaga, E., Im, S. A., Lee, K. S., Hurvitz, S. A., Cortes, J., Lee, C., … DESTINY-Breast01 Investigators (2020).
Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. The New England Journal of Medicine, 382(7), 610–621.
https://doi.org/10.1056/NEJMoa1914510. - Bachelot, T., Romieu, G., Campone, M., Diéras, V., Cropet, C., Dalenc, F., Jimenez, M., Le Rhun, E., Pierga, J. Y., Gonçalves, A.,
Leheurteur, M., Domont, J., Gutierrez, M., Curé, H., Ferrero, J. M., & Labbe-Devilliers, C. (2013). Lapatinib plus capecitabine in
patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2
study. The Lancet. Oncology, 14(1), 64–71. https://doi.org/10.1016/S1470-2045(12)70432-1. - Lin, N. U., Freedman, R. A., Ramakrishna, N., Younger, J., Storniolo, A. M., Bellon, J. R., Come, S. E., Gelman, R. S., Harris, G. J.,
Henderson, M. A., Macdonald, S. M., Mahadevan, A., Eisenberg, E., Ligibel, J. A., Mayer, E. L., Moy, B., Eichler, A. F., &
Winer, E. P. (2013). A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor
Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Research and Treatment, 142(2), 405–
414. https://doi.org/10.1007/s10549-013-2754-0 - Bartsch, R., Berghoff, A., Pluschnig, U., Bago-Horvath, Z., Dubsky, P., Rottenfusser, A., DeVries, C., Rudas, M., Fitzal, F.,
Dieckmann, K., Mader, R. M., Gnant, M., Zielinski, C. C., & Steger, G. G. (2012). Impact of anti-HER2 therapy on overall
survival in HER2-overexpressing breast cancer patients with brain metastases. British Journal of Cancer, 106(1),
25–31.https://doi.org/10.1038/bjc.2011.531. - Pivot, X., Manikhas, A., Żurawski, B., Chmielowska, E., Karaszewska, B., Allerton, R., Chan, S., Fabi, A., Bidoli, P., Gori, S.,
Ciruelos, E., Dank, M., Hornyak, L., Margolin, S., Nusch, A., Parikh, R., Nagi, F., DeSilvio, M., Santillana, S., Swaby, R. F., …
Semiglazov, V.(2015). CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus
Trastuzumab Plus Capecitabine in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer.
Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 33(14), 1564–1573. https://
doi.org/10.1200/JCO.2014.57.1794 - Freedman, R. A., Gelman, R. S., Anders, C. K., Melisko, M. E., Parsons, H. A., Cropp, A. M., Silvestri, K., Cotter, C. M., Componeschi,
K. P., Marte, J. M., Connolly, R. M., Moy, B., Van Poznak, C. H., Blackwell, K. L., Puhalla, S. L., Jankowitz, R. C., Smith, K. L.,
Ibrahim, N., Moynihan, T. J., O'Sullivan, C. C., … Translational Breast Cancer Research Consortium (2019). TBCRC 022: A Phase II
Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain
Metastases. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 37(13), 1081–1089. https://
doi.org/10.1200/JCO.18.01511 - Awada, A., Colomer, R., Inoue, K., Bondarenko, I., Badwe, R. A., Demetriou, G., Lee, S. C., Mehta, A. O., Kim, S. B., Bachelot, T.,
Goswami, C., Deo, S., Bose, R., Wong, A., Xu, F., Yao, B., Bryce, R., & Carey, L. A. (2016). Neratinib Plus Paclitaxel vs Trastuzumab
Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer: The NEfERT-T Randomized Clinical Trial. JAMA
Oncology, 2(12), 1557–1564. https://doi.org/10.1001/jamaoncol.2016.0237 - Saura, C., Oliveira, M., Feng, Y. H., Dai, M. S., Chen, S. W., Hurvitz, S. A., Kim, S. B., Moy, B., Delaloge, S., Gradishar, W., Masuda,
N., Palacova, M., Trudeau, M. E., Mattson, J., Yap, Y. S., Hou, M. F., De Laurentiis, M., Yeh, Y. M., Chang, H. T., Yau, T., … NALA
Investigators (2020). Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer
Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. Journal of Clinical Oncology : Official Journal of the
American Society of Clinical Oncology, 38(27), 3138–3149. https://doi.org/10.1200/JCO.20.00147 - Murthy, R. K., Loi, S., Okines, A., Paplomata, E., Hamilton, E., Hurvitz, S. A., Lin, N. U., Borges, V., Abramson, V., Anders, C., Bedard,
P. L., Oliveira, M., Jakobsen, E., Bachelot, T., Shachar, S. S., Müller, V., Braga, S., Duhoux, F. P., Greil, R., Cameron, D., … Winer, E.
P.(2020). Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. The New England Journal of
Medicine, 382(7), 597–609. https://doi.org/10.1056/NEJMoa1914609 - Borges, V. F., Ferrario, C., Aucoin, N., Falkson, C., Khan, Q., Krop, I., Welch, S., Conlin, A., Chaves, J., Bedard, P. L., Chamberlain, M.,
Gray, T., Vo, A., & Hamilton, E. (2018). Tucatinib Combined With Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive
Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA oncology, 4(9), 1214–1220. https://doi.org/10.1001/jamaoncol.2018.1812 - Ferraro, E., Barrio, A.V. , Patil, S., Robson, M. E., Dang, C. T. (2020) Incidence of brain metastases in patients receiving
neoadjuvant chemotherapy (NAC) with trastuzumab and pertuzumab (HP) in HER2-positive early breast cancer (BC). Journal of
Clinical Oncology : Official Journal of the American Society of Clinical Oncology, 38(15)_suppl. https://doi.org/10.1200/
JCO.2020.38.15_suppl.e12653. - von Minckwitz, G., Huang, C. S., Mano, M. S., Loibl, S., Mamounas, E. P., Untch, M., Wolmark, N., Rastogi, P., Schneeweiss, A.,
Redondo, A ., Fischer, H. H., Jacot, W., Conlin, A. K., Arce-Salinas, C., Wapnir, I. L., Jackisch, C., DiGiovanna, M. P., Fasching, P. A.,
Crown, J. P., Wülfing, P., … KATHERINE Investigators (2019). Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast
Cancer. The New England Journal of Medicine, 380(7), 617–628. https://doi.org/10.1056/NEJMoa1814017. - Subbiah, I. M., Lei, X., Weinberg, J. S., Sulman, E. P., Chavez-MacGregor, M., Tripathy, D., Gupta, R., Varma, A., Chouhan, J.,
Guevarra, R. P., Valero, V., Gilbert, M. R., & Gonzalez-Angulo, A. M. (2015). Validation and Development of a Modified Breast Graded
Prognostic Assessment As a Tool for Survival in Patients With Breast Cancer and Brain Metastases. Journal of Clinical Oncology:
Official Journal of the American Society of Clinical Oncology, 33(20), 2239–2245. https://doi.org/10.1200/JCO.2014.58.8517 - Criscitiello, C., André, F., Thompson, A. M., De Laurentiis, M., Esposito, A., Gelao, L., Fumagalli, L., Locatelli, M., Minchella, I., Orsi,
F., Goldhirsch, A., & Curigliano, G. (2014). Biopsy confirmation of metastatic sites in breast cancer patients: clinical impact and future
perspectives. Breast Cancer Research: BCR, 16(2), 205. https://doi.org/10.1186/bcr3630 - Cardoso, F., Senkus, E., Costa, A., Papadopoulos, E., Aapro, M., André, F., Harbeck, N., Aguilar Lopez, B., Barrios, C. H., Bergh,
J., Biganzoli, L., Boers-Doets, C. B., Cardoso, M. J., Carey, L. A., Cortés, J., Curigliano, G., Diéras, V., El Saghir, N. S., Eniu, A.,
Fallowfield, L., … Winer, E. P. (2018). 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)†.
Annals of Oncology: Official Journal of the European Society for Medical Oncology, 29(8), 1634–1657. https://doi.org/10.1093/
annonc/mdy192.
