![]()
Biomedical Sciences, Biomed Biopharm Res., 2021; 18(1):38-47
doi: 10.19277/bbr.18.1.257; download the pdf version [+] here
Topically applied methyl nicotinate evokes a temporary inflammation on human skin
Sérgio Faloni de Andrade*, Clemente Rocha, Luis Monteiro Rodrigues
Universidade Lusófona - CBIOS - Research Center for Biosciences and Health Technologies, Av. Campo Grande, 376, 1749-024, Lisboa, Portugal
*corresponding author:
Abstract
Some challengers such as methylnicotinate (MN) have been used in human models to study the anti-inflammatory effect of topical formulations. However, MN skin responses are still poorly understood and widely varied. In the present study we aim to contribute to better characterise those responses. Eight healthy participants were selected. All procedures were approved by the institutional Ethics Committee. Two aqueous MN dilutions (0.5% and 1.0%) were left in contact for 1 minute in the anterior forearm skin. Following exposure, skin reactions were clinically and biometrically assessed at 30, 60 and 120 minutes and compared with baseline. Measurements involved the ICDRG clinical score scale and select analytical technologies - laser Doppler flowmetry, Polarised Spectroscopy, Transepidermal Water Loss Meter, and High Resolution Sonography. Results have shown that MN application evoked a maximal response at 30 minutes with an increase in the ICDRG score between 1-2. Significant changes in TEWL and microcirculation were observed, as was an increased dermal hypoecogenicity (edema), detected by HRS. These effects are compatible with a localised short-duration inflammation and reinforce the interest of MN to be used as a safe and controllable challenger in human models.
Keywords: Methyl Nicotinate, in vivo human skin, microcirculation, TEWL, inflammation
Received: 14/04/2021; Accepted: 31/05/2021
Introduction
Inflammation is a key feature in clinical dermatology, with an enormous variety of factors and mechanisms affecting skin inflammatory processes and their clinical expression in terms of signs and subjective symptoms. These complicated interactions between skin and the immune system and skin and the nervous system, sometimes referred to as the neuro‐immuno‐cutaneous (NIC) system are probably a major component of this reality still far from being fully understood (1-3). From the therapeutic point of view this is a remarkable target for pharma and skin care industries since inflammatory disorders are present during the whole human lifecycle, from the newborn to the elderly (4-6). Market demand is growing widely, such that “over-the-counter” medicines and cosmetics are among the consumers’ preference all over the world, reflecting also easy access and product satisfaction (7,8).
The systematic investment in the safety evaluation of dermatological products before market introduction partially explains this success. In the post-animal-ban testing era, many new approaches for skin irritation testing and risk assessment were proposed in vitro, primarily in human (9,10). Several challengers were progressively developed to assess, again in human, the effectiveness of anti-inflammatory formulations, such as the anionic detergent sodium lauryl sulfate (SLS), benzoic acid, sorbic acid, methyl nicotinate (MN). Some mechanical approaches (friction, abrasive grains, tape-striping) have also been proposed and used as irritants (11,12). A remarkable amount of experimental information accumulated around SLS and MN have led these compounds to be regarded as safe and capable of reproducing a controlled “inflammation-like” state useful for safety and efficacy assessments of many topical products (12-15). However, data shows that SLS is a strong skin irritant which deforms cell membrane proteins, especially when applied under occlusion (0.5% dilutions and higher), provoking an intense, clinically variable, and lasting aggression to the skin not without risk or/and discomfort. (16-18). In turn, MN is a nicotinic acid derivative whose vasodilator capacity seems to be of multifactorial origin (19,20). MN, as benzoic acid, sorbic acid, and nicotinic acid derivatives, is able to provoke nonimmunologic contact urticaria (NICU), the most common form of immediate contact reaction resulting in a rapid inflammatory response without the requirement of previous sensitization. Both challengers (SLS and MN) have also been long questioned for reproducibility (11). Their mechanisms of action and factors that condition individual reactions have not been completely identified (20-23).
Nevertheless, these models have added an enormous amount of valuable scientific knowledge for physiological-pharmacological and toxicological research, even if still far from being complete. In the present study, we try to look further into the action mechanisms of MN in human in vivo skin using different non-invasive technologies.
Materials and Methods
Participants
Eight healthy individuals, both sexes (three men and five women), ages between 20 and 59 years old (mean 41.37 ± 11.32 years old) were selected according to previously defined inclusion/non-inclusion criteria, namely (i) no visible cutaneous lesions and no past or present record of dermatological disease or atopy, (ii) no application of any cosmetics in the test area 48 hours prior to the study at baseline, and (iii) absence of any pharmacological treatment that might interfere with measurements All procedures observed the principles of good clinical practice of the Helsinki Declaration and respective amendments (24) and included informed written consent. The study was previously approved by the institutional Ethical Committee.
Experimental
Two aqueous MN dilutions of 0.5% or 1.0% were randomly applied (in the form of soaked 5 mm diameter paper discs) in pre-defined areas (1cm2) of the anterior aspect of the forearm with no occlusion. The dilutions remained in contact with the skin for 1 minute before removal. A similar empty area in the same forearm was used as a negative control. Skin functional variables were measured by non-invasive technologies at time 0 (control) and after MN contact at 30, 60, and 120 minutes.
Analytical technologies for the evaluation of the effects of exposure were:
- Polarized Spectroscopy using the Tissue Viability Imaging system (TiVi 700, WheelsBridge AB, Linköping, Sweden), which quantifies the concentration of red blood cells (CRBC - TiVi index), expressed in arbitrary units, in a previously selected region of interest (ROI);
- Laser Doppler Flowmetry (LDF, PeriFlux System 5000, Perimed AB, Järfälla, Sweden), which measures the perfusion expressed in arbitrary units of perfusion (PUs);
- Transepidermal Water Loss Meter, a direct indicator of “epidermal barrier” integrity, measured by evaporimetry (Tewameter TM300, CK electronics, Köln, Germany) and expressed as g/hour/m2.
- High-resolution sonography (HRS) (Dermascan C, Cortex Technology, Hadsund, Denmark) providing a bidimensional color image recorded at a velocity of 1580 m/s, using a 20MHz probe placed on the skin in a fixed standard position (25). The color image was converted into a greyscale image for further analysis and processed by software ImageJ® (NIH, Bethesda, Maryland, USA).
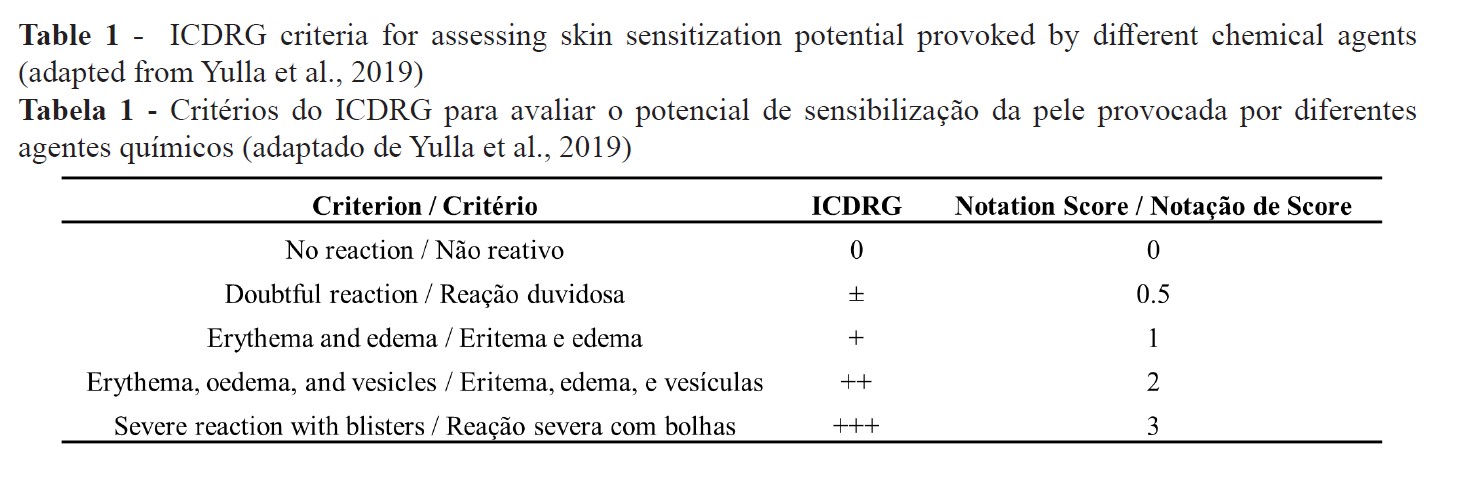
Skin responses were also scored by direct observation using the International Contact Dermatitis Group Research (ICDRG) scale (26) adapted by Yulla et al., (3) (Table 1).
All measurements took place in a controlled humidity and temperature environment (humidity ~50%, temperature 21 + 2 ºC) where participants were allowed to acclimatize for a minimum of 30 minutes prior to evaluations.
Statistics
Data reported as mean ± standard error of the mean (SEM) were compared by the Wilcoxon signed-rank test using GraphPadPrism ٥® software (GraphPad software, San Diego, CA, USA). A p value < 0.05 was considered significant in all experiments.
Results and Discussion
The clinical evaluation with the ICDRG score scale revealed that both MN dilutions significantly increased the score after 30 minutes (1.5 ± 0.19 and 1.37 ± 0.18 for 0.5% and 1.0%, respectively). The reaction was visually much more intense at 30 minutes, decreasing almost to the baseline after 60 and 120 minutes (Figure 1).
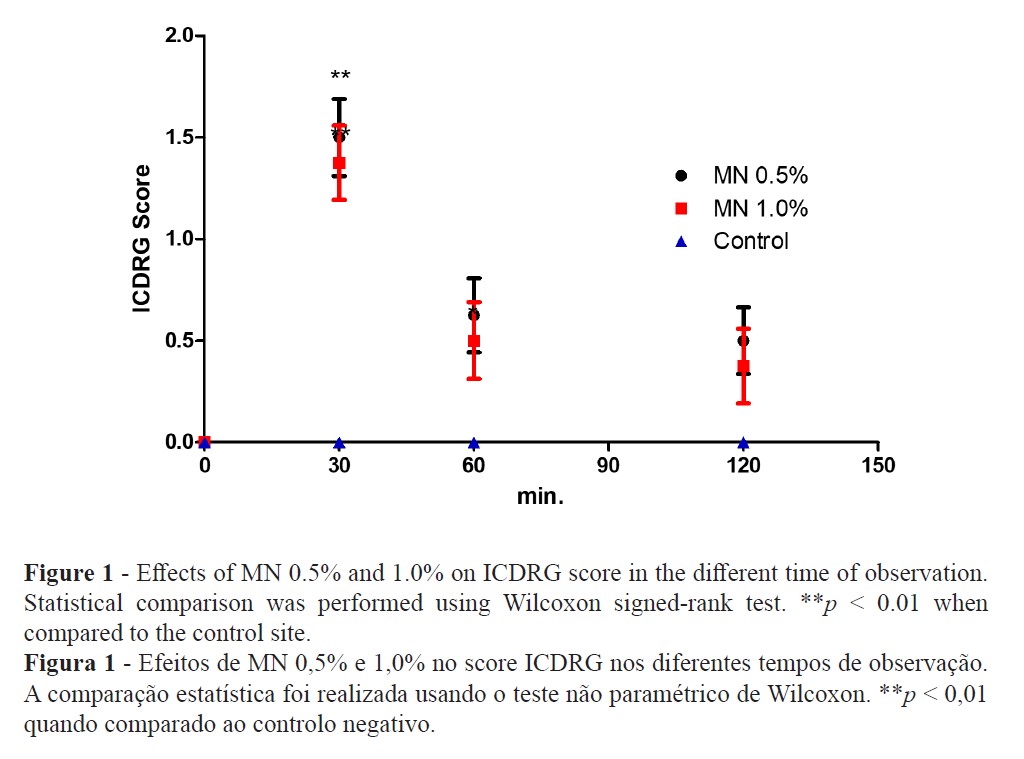
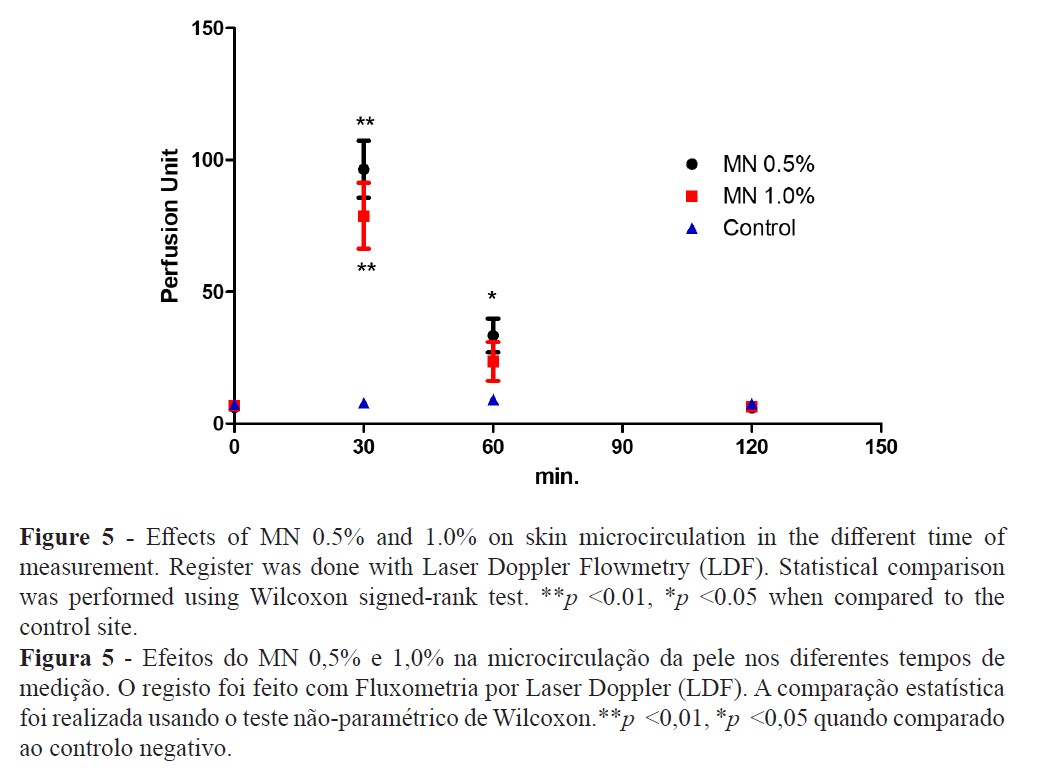
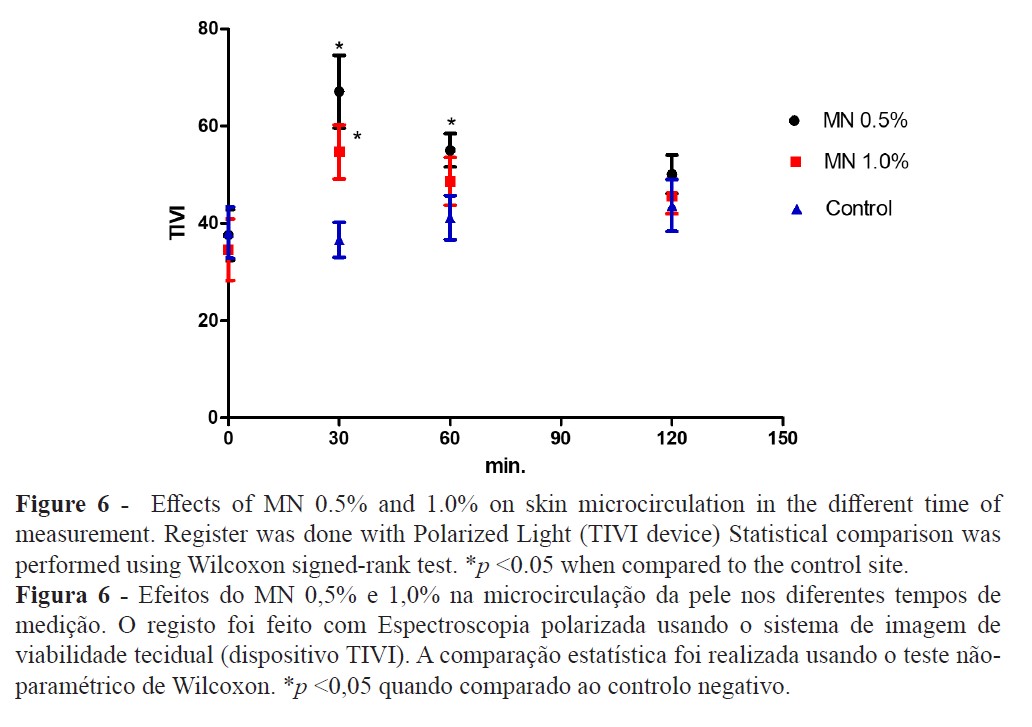
The TEWL increase suggests that MN disturbed the epidermal skin barrier function (Figure 2) while high-resolution sonography (HRS) revealed an increased dermal hypoecogenicity which is suggestive of edema formation (Figures 3 and 4). The skin barrier changes were expected and have been previously reported (27). However, the presence of edema, confirming a cardinal sign of inflammation, as detected by HRS images, have not been previously described under the present experimental conditions. Consistent skin microcirculatory changes were also detected by both LDF and TIVI systems (Figures 5 and 6).
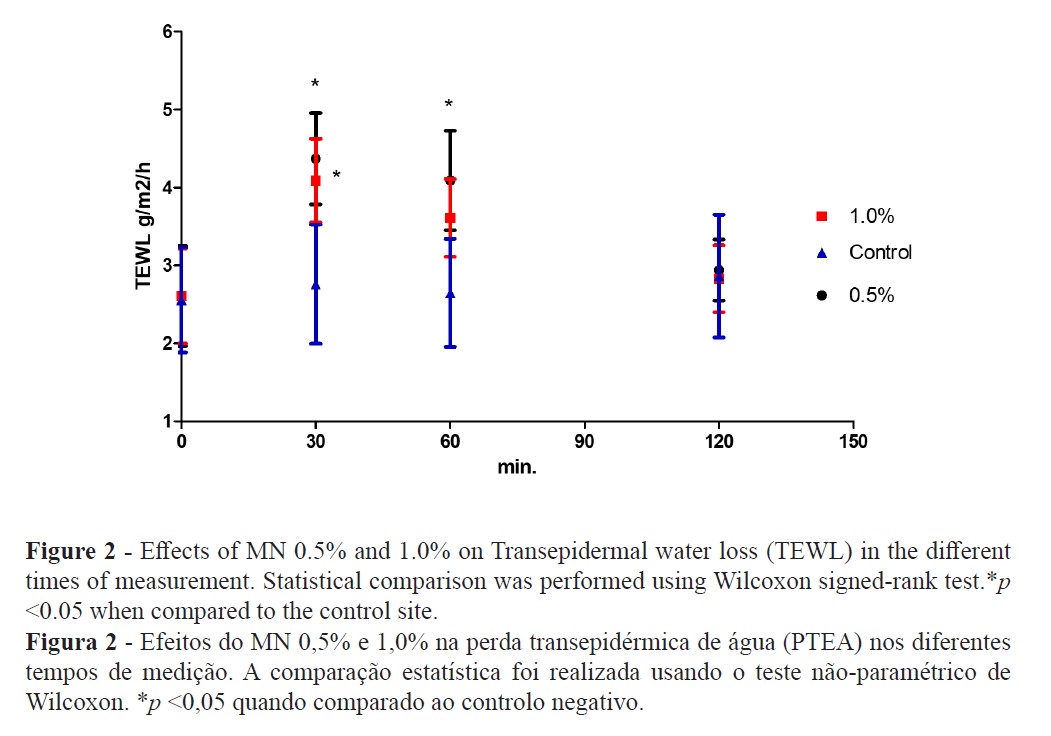
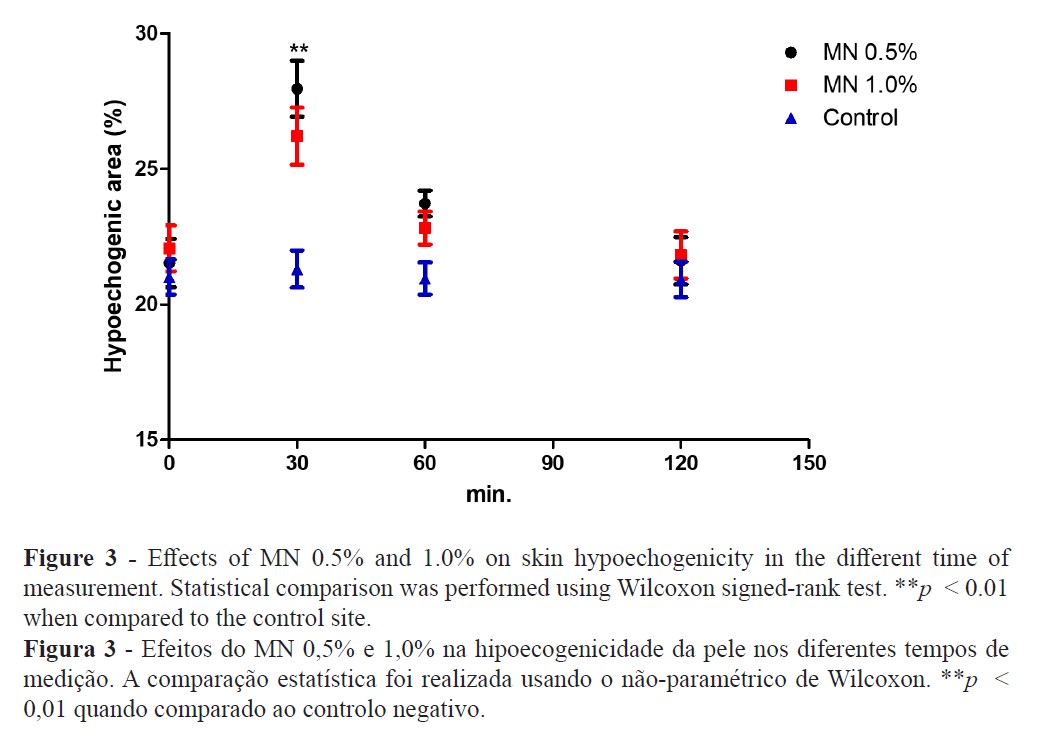
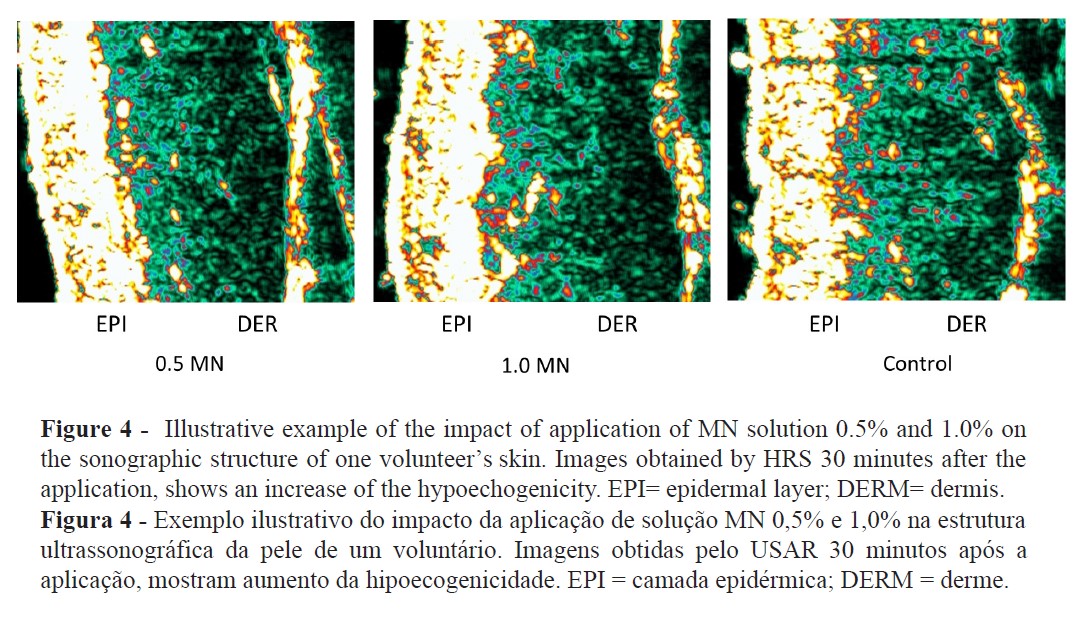
Skin irritation and inflammation are common components of the dermatology glossary used to describe responses to a wide variety of chemical, physical, or biological stimuli. Often its significance is not very clear, being conversely used in distinct clinical processes. Skin barrier disruption, induction of a cytokine cascade, and involvement of oxidative stress are the main pathological alterations observed after cutaneous aggression resulting in a visible or subclinical inflammatory reaction (12,28). Additionally, a strong interindividual variability response to irritants is recognised, but when there is sufficient exposure and high concentrations of the irritant, all individuals are prone to develop this cutaneous reaction (12,28,29).
Our approach suggests that MN application evokes a consistent change of all the skin, from the epidermal barrier to the dermal plexus. This vasodilation is known, described as painless, short-term, and reproducible when evaluated in the same person using the same dose and application site (30). However, the peak response for this vasodilation and, all other parameters as well, occurred at 30 minutes after MN application and, although not significant, we noticed a trend for the highest response at the lowest concentration (MN 0.5% solution). These results are inline with similar observations (31,32) but the opposite has also been described (11). The presence of a localised edema, only detected by the HRS, suggests a short duration inflammatory process that might be mediated by prostaglandins with neurogenic components since histamine and nitric oxide might not be involved in this process (15,31).
Conclusion
Our results contribute to a better understanding of the impact of MN on human skin and its interest as an experimental challenger. Even in low concentrations, MN is capable of modifying the entire cutaneous structure for a short period of time. This seems to be particularly suitable for safety and efficacy studies involving the anti-inflammatory activity. Nevertheless, more studies are needed to better understand and control MN mechanisms of action.
Authors Contributions Statement
LMR and SFA conceptual design; SFA and CR experimental and data analysis; SFA and LMR drafted and wrote the manuscript in its final version.
Acknowledgements
This research is funded by Fundação para a Ciência e a Tecnologia (FCT) through grant UIDB/04567/2020 to CBIOS. Sérgio Faloni de Andrade is funded by Foundation for Science and Technology (FCT) - Scientific Employment Stimulus contract with the reference number CEEC/CBIOS/PMHD/2018.
Conflict of Interests
Editors involved in this manuscripts’ authorship had no participation in the review or decision process. All authors have stated that there are no financial and/or personal relationships that could represent a potential conflict of interest.
References
- Kanitakis, J. (2002). Anatomy, histology and immunohistochemistry of normal human skin. European Journal of Dermatology, 12(4), 390-401.
- Hänel, K., Cornelissen, C., Lüscher, B., & Baron, J. (2013). Cytokines and the Skin Barrier. International Journal of Molecular Sciences, 14(4), 6720-6745. doi: 10.3390/ijms14046720
- Vidal Yucha, S., Tamamoto, K., & Kaplan, D. (2019). The importance of the neuro‐immuno‐cutaneous system on human skin equivalent design. Cell Proliferation, 52(6), e12677. doi: 10.1111/cpr.12677
- Scrivo, R., Vasile, M., Bartosiewicz, I., & Valesini, G. (2011). Inflammation as “common soil” of the multifactorial diseases. Autoimmunity Reviews, 10(7), 369-374. doi: 10.1016/j.autrev.2010.12.006
- Medzhitov, R. (2008). Origin and physiological roles of inflammation. Nature, 454(7203), 428-435. doi: 10.1038/nature07201
- de Almeida Roediger, M., de Fátima Nunes Marucci, M., Duim, E., Santos, J., de Oliveira Duarte, Y., & de Oliveira, C. (2019). Inflammation and quality of life in later life: findings from the health, well-being and aging study (SABE). Health And Quality Of Life Outcomes, 17(1),26. doi: 10.1186/s12955-019-1092-2
- Milam, E., & Rieder, E. (2021). An Approach to Cosmeceuticals. Essential Psychiatry For The Aesthetic Practitioner, 42-48.
- Tetali, B., Fahs, F., & Mehregan, D. (2019). Popular over‐the‐counter cosmeceutical ingredients and their clinical efficacy. International Journal Of Dermatology, 59(4), 393-405. doi: 10.1111/ijd.14718
- Hoffmann, S., Kleinstreuer, N., Alépée, N., Allen, D., Api, A., & Ashikaga, T. et al. (2018). Non-animal methods to predict skin sensitization (I): the Cosmetics Europe database. Critical Reviews In Toxicology, 48(5), 344-358. doi: 10.1080/10408444.2018.1429385
- Robinson, M., Cohen, C., de Fraissinette, A., Ponec, M., Whittle, E., & Fentem, J. (2002). Non-animal testing strategies for assessment of the skin corrosion and skin irritation potential of ingredients and finished products. Food And Chemical Toxicology, 40(5), 573-592. doi: 10.1016/s0278-6915(02)00005-4
- Jumbelic, L., Liebel, F., & Southall, M. (2006). Establishing a Minimal Erythema Concentration of Methyl Nicotinate for Optimum Evaluation of Anti-Inflammatories. Skin Pharmacology And Physiology, 19(3), 147-152. doi: 10.1159/000092595
- Fluhr, J., Akengin, A., Bornkessel, A., Fuchs, S., Praessler, J., & Norgauer, J. et al. (2005). Additive impairment of the barrier function by mechanical irritation, occlusion and sodium lauryl sulphate in vivo. British Journal Of Dermatology, 153(1), 125-131. doi: 10.1111/j.1365-2133.2005.06430.x
- Tupker, R., Willis, C., Berardksca, E., Lee, C., Fartasch, M., Atinrat, T., & Serup, J. (1997). Guidelines on sodium lauryl sulfate (SLS) exposure tests. Contact Dermatitis, 37(2), 53-69. doi: 10.1111/j.1600-0536.1997.tb00041.x
- Tadić, V., Arsić, I., Zvezdanović, J., Zugić, A., Cvetković, D., & Pavkov, S. (2017). The estimation of the traditionally used yarrow ( Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. Journal Of Ethnopharmacology, 199, 138-148. doi: 10.1016/j.jep.2017.02.002
- Elawa, S., Mirdell, R., Farnebo, S., & Tesselaar, E. (2019). Skin blood flow response to topically applied methyl nicotinate: Possible mechanisms. Skin Research And Technology, 26(3), 343-348. doi: 10.1111/srt.12807
- Lee, C., Kim, H., Han, H., & Park, C. (2004). A Comparison Study of Nonanoic Acid and Sodium Lauryl Sulfate in Skin Irritation. Exogenous Dermatology, 3(1), 19-25. doi:10.1159/000084139
- Gabard, B., Chatelain, E., Bieli, E., & Haas, S. (2001). Surfactant irritation: in vitro corneosurfametry and in vivo bioengineering. Skin Research And Technology, 7(1), 49-55. doi: 10.1034/j.1600-0846.2001.007001049.x
- Pinto, PC., Martinho, H., & Rodrigues L.M. (2007) Studying the influence of the phototype over human skin’s response to Sodium Lauril Sulfate contact in vivo, Revista Saúde & Tecnologia, (4)1, 47-56.
- Morrow, J., Parsons, W., & Roberts, L. (1989). Release of markedly increased quantities of prostaglandin D2 in humans following the administration of nicotinic acid. Prostaglandins, 38(2), 263-274. doi: 10.1016/0090-6980(89)90088-9
- Guy, R., & Maibach, H. (1982). Rapid radial transport of methyl nicotinate in the dermis. Archives Of Dermatological Research, 273(1-2), 91-95. doi: 10.1007/BF00509031
- Guy, R., Tur, E., Bjerke, S., & Maibach, H. (1985). Are there age and racial differences to methyl nicotinate—induced vasodilatation in human skin? Journal Of The American Academy Of Dermatology, 12(6), 1001-1006. doi: 10.1016/s0190-9622(85)70128-4
- Leoty-Okombi, S., Gillaizeau, F., Leuillet, S., Douillard, B., Le Fresne-Languille, S., & Carton, T. et al. (2021). Effect of Sodium Lauryl Sulfate (SLS) Applied as a Patch on Human Skin Physiology and Its Microbiota. Cosmetics, 8(1), 6. doi: 10.3390/cosmetics8010006
- De Jongh, C., Verberk, M., Withagen, C., Jacobs, J., Rustemeyer, T., & Kezic, S. (2006). Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Dermatitis, 54(6), 325-333. doi: 10.1111/j.0105-1873.2006.00848.x.
- World Medical Association Declaration of Helsinki. (2013). JAMA, 310(20), 2191. doi: 10.1001/jama.2013.281053
- Seidenari, S., Nakijo, A., Pepe, P., & Giannetti, A. (1991). Ultrasound B scanning with image analysis for assessment of allergic patch test reactions. Contact Dermatitis, 24(3), 216-222. doi: 10.1111/j.1600-0536.1991.tb01701.x
- Marzulli, F., & Maibach, H. (1976). Contact allergy: Predictive testing in man. Contact Dermatitis, 2(1), 1-17. doi: 10.1111/j.1600-0536.1976.tb02972.x
- Ratz-lyko, A., Arct, J., & Pytkowska, K. (2016). Moisturizing and antiinflammatory properties of cosmetic formulations containing Centella asiatica extract. Indian Journal Of Pharmaceutical Sciences, 78(1), 27. doi: 10.4103/0250-474x.180247
- Fluhr, J., Darlenski, R., Angelova-Fischer, I., Tsankov, N., & Basketter, D. (2008). Skin Irritation and Sensitization: Mechanisms and New Approaches for Risk Assessment. Skin Pharmacology And Physiology, 21(3), 124-135. doi: 10.1159/000135635
- Sasseville, D. (2008). Occupational Contact Dermatitis. Allergy, Asthma, And Clinical Immunology, 4(2), 59-65. doi: 10.1186/1710-1492-4-2-59
- Poelman, M., Piot, B., Guyon, F., Deroni, M., & Leveque, J. (1989). Assessment of topical non-steroidal anti-inflammatory drugs. Journal Of Pharmacy And Pharmacology, 41(10), 720-722. doi: 10.1111/j.2042-7158.1989.tb06350.x
- Vertuani, S., Ziosi, P., Solaroli, N., Buzzoni, V., Carli, M., & Lucchi, E. et al. (2003). Determination of antioxidant efficacy of cosmetic formulations by non-invasive measurements. Skin Research And Technology, 9(3), 245-253. doi: 10.1034/j.1600-0846.2003.00018.x
