![]()
Biopharmaceutical Sciences, Biomed Biopharm Res., 2021; 18(1):68-82
doi: 10.19277/bbr.18.1.251; download pdf version [+] here
Type 1 medication review based on a pharmacy’s electronic medication records: first steps towards an algorithm to stratify patients for tailored pharmacy services
Ligia Reis 1,2, Miguel Monteiro 2, Luis Lourenço 1, João Gregório 1*
1CBIOS – Universidade Lusófona’s Research Center for Biosciences & Health Technologies, Campo Grande 376, 1749-024 Lisboa, Portugal; 2 Farmácia Central do Cacém, Praceta Aquilino Ribeiro, 5/6 – r/c, 2735-060 Agualva-Cacém, Portugal
*corresponding author:
Abstract
Algorithms, queries, and knowledge-based systems are among approaches to screen electronic patient records stored in databases and support pharmacist medication reviews. The aim of this study was to perform a type 1 medication review and identify clusters that enable the definition of an algorithm to tailor pharmacy professional intervention. A retrospective observational study was conducted on a convenience sample of pharmacy records. Records were included if patients had a medication dispensing history between June 2017 - July 2018 and used two or more chronic medications. Statistical analysis used a two-step cluster to identify common characteristics among fifty-five sets of patient records which underwent Type 1 medication review. The median number of drugs used per patient was five [IQR: 3.0 – 7.0]. 18.2% of patients had inappropriate drugs, and 30.9% had moderate or major interaction potential. Four clusters were identified based on the variables of interactions, number of drugs used, contraindications, Beers criteria and measurable biomarkers, allowing to envision possible pharmaceutical interventions, as well as the priority in providing that intervention. The identification of patient clusters via medication review of electronic records of pharmacy patients supports the design of criteria-based algorithms, likely to be automated.
Keywords: Pharmaceutical services; medication review; electronic health records; algorithms
Received: 11/09/2020; Accepted: 06/02/2021
Introduction
As the burden of Non-Communicable Diseases (NCD) increases globally, chronic disease management has become central to every health system (1). Among several models to deal with this threat, the Chronic Care Model has emerged as an interprofessional collaboration model suited to the challenges brought by NCD’s high burden on health systems (2). This model has in turn provided the context to the rethinking of health professionals’ roles and duties (3). Community pharmacists have joined this movement after the emergence of pharmaceutical care concepts and the implementation of several projects that showcased community pharmacists as effective primary care professionals (4).
Since the 1990s, medication reviews were included in community pharmacy practice. Pharmacist-led medication review services are available in Europe (UK, NL, BE, SE, DK), United States of America (USA), Australia, Canada and New Zealand (5,6). The Pharmaceutical Care Network Europe (PCNE) defines medication review as “a structured evaluation of patients’ medications with the aim of optimizing medication use and improving health outcomes”. This entails detecting drug related problems (DRPs) and recommending interventions (7). Four types of review are identified depending on the patient information available to the pharmacist (Table 1). All types of Medication reviews are always constituted by a diagnostic intervention that aims to identify DRPs. All but type 1 require the patient to be present at the point of care, both to gather important information and to provide an educational intervention to support patient knowledge and adherence (8).
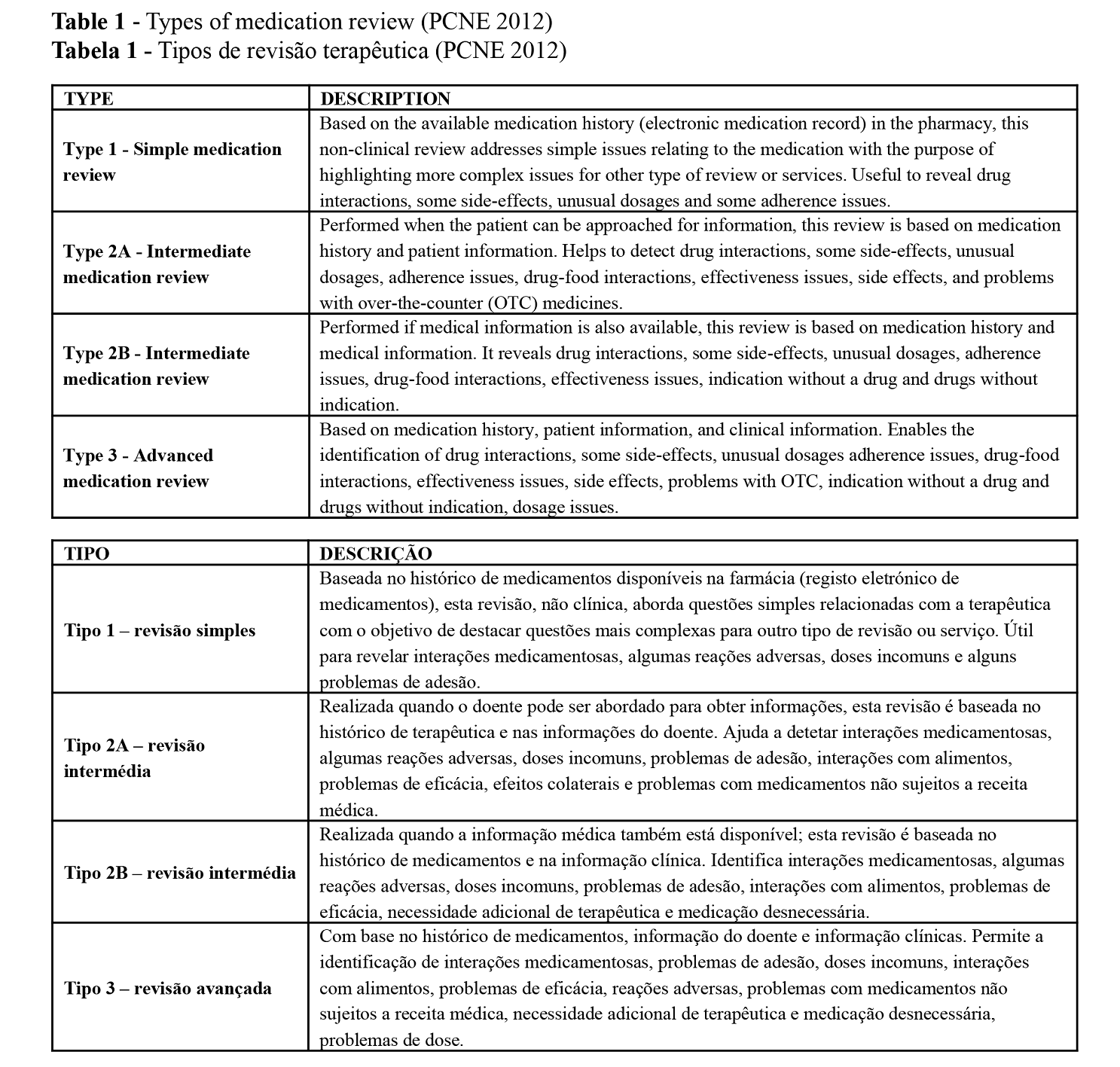
When provided, medication reviews resulted in a decrease in the number of drug-related problems, more changes in medication, more drugs with decreased dosage, and a greater decrease or smaller increase in the number of drugs (6). The reviews also have a positive benefit on patient outcomes specifically on the attainment of clinical biomarkers goals (5), and improvement of process outcomes such as reduced polypharmacy, more appropriate formulations, and more appropriate choices of medications (8). The appropriateness of medications can also be assessed with different tools. Among them, Beers’ criteria is one of the most commonly used (9), assisting healthcare providers in improving medication safety in older adults by identifying medications for which the risks of their use outweigh the benefits (10).
In spite of being a service that can enhance the pharmacists’ role in the health system, the implementation of medication reviews has not been thoroughly achieved. Major barriers to implement an effective medication review service cited in the literature have been lack of time, collaboration with physicians, pharmacists’ self-confidence, and patient inclusion (11,12). Therefore, there is a need to enhance the efficiency of medication review services. The use of IT may unlock this potential efficiency (13), allowing more time to augment medication review provision and the development of new services.
The inception of the Digital Age in the 1960s, with the development of electronics, information technology (IT) and automated production, has shaped the practice of pharmacy (14). Nowadays, community pharmacy practice is highly computerized, collecting patient information on a continuous basis and creating a permanently updated electronic healthcare record (EHR) (15). Electronic healthcare records provide real-time access to patient information, such as the patient’s medical condition, visits to healthcare providers, and images and reports of diagnostic procedures, thus enabling a complete longitudinal record of evidence-based care (15). These EHR can impact decision-making, how healthcare professionals and patients interact with each other, how health information is stored and used, and how patients manage their health themselves through electronic apps and devices (16).
For community pharmacists, EHRs facilitate medication reviews and the identification of drug therapy problems (16). Structured methods and appropriate software are important tools to increase pharmacist effectiveness and improve health outcomes (17,18). Databases, that store patients' electronic records can be queried using search algorithms, for example, to select non-adherent patients or patients receiving specific medications (17), or to identify inpatients at risk of drug-related problems (19). Algorithms are known to support medication review processes identifying and discontinuing potentially inappropriate medications, which may otherwise lead older population to harmful events (20). Algorithms are liable to be automated and therefore able to support a continuous, reproducible, and less time-consuming medication review (21).
However, the definition of an algorithm tailored to the Portuguese pharmacies’ information system is lacking. In order to design an algorithm, criteria must be defined based on the variables available on patient information of electronic records. Therefore, the aim of this study was to apply a type 1 medication review to a sample of patients’ electronic medication records of a community pharmacy. This medication review will then enable the identification of clusters of patients in need of additional pharmacy professional interventions, a first step toward the design of a new decision algorithm.
Materials and Methods
An exploratory retrospective observational study was conducted performing a type 1 medication review to electronic patient records. A convenience sample was used, extracted from the database of electronic medication records of a community pharmacy located in the district of Lisbon. The following inclusion criteria were used: records of continuous therapy in 12 months (June 2017-July 2018); use of two or more chronic prescription medications. Continuous therapy was defined has having records for medication dispensation fulfilling the expected duration of therapy without interruption.
To perform medication reviews, the criteria of necessity, effectiveness and safety of the 2nd Granada Consensus were applied (22). Drug safety was assessed with Sifarma™ software and included determination of interactions and contraindications, and respective level of severity. Degree of severity was classified as minor, moderate or major. Sifarma™ software enables to register and access patients’ dispensing history, biomarkers, interactions, contraindications, and adverse reactions (23). Potentially inappropriate medications (PIM) were identified applying the Table 2 of Beers criteria 2015 (24), to patients over 65 years of age, classifying each drug as appropriate or inappropriate. This table lists potentially inappropriate medications for older adults outside the palliative care and hospice setting, including medications to avoid for many or most older adults, even in the absence of patient’s diagnosis (25). These criteria were considered as not applicable for patients under 65 years old. Duplication of the same active substance or same therapeutic class was also identified. The Summary of Product Characteristics (SPC) of each medication was also consulted to identify the need for monitoring and which parameters to evaluate.
Statistical analysis of the data was performed using IBM SPSS version 22 and Microsoft Excel. Significance level was set to 5%. Non-parametric tests were performed because there was no normal distribution in all continuous variables. For categorical and ordinal variables, Spearman correlations and Chi-Square tests were used. Two-step cluster analysis was performed to identify the number of possible clusters within the sample. Patients had to sign an informed consent to have their record stored at the pharmacy, where consent was given to use anonymized data for research purposes. No further consent was deemed necessary.
Results
The final sample of electronic records eligible for type 1 medication review included 55 patients. 54.5% were female and 45.5% were male. Mean age of the sample was 65.67 years (SD 14.8) with a median of 68 years [IQR: 55.0 – 77.0]. Age had a normal distribution in women but not in men (Shapiro-Wilk; p = 0.634 women; p = 0.017 men). In this sample, men were on average older than women, but the differences were not significant (Mann-Whitney; p = 0.076) (Table 2). The age variable was then categorized with a cut-off in the Beers criteria (< 65 years; ≥ 65 years).
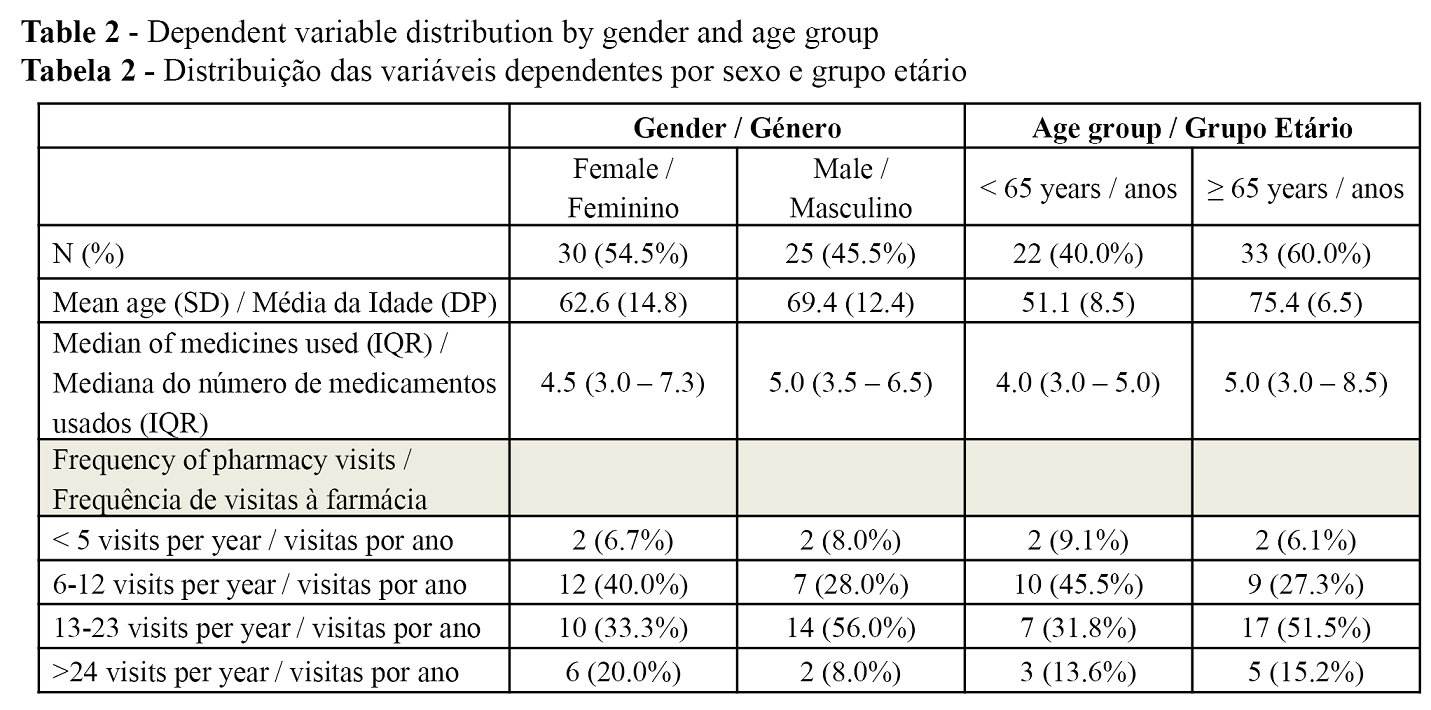
The results revealed that 58.1% of patients in the sample went to the pharmacy at least once a month. Differences between genders in frequency of pharmacy visits were not significant (p = 0.313). Despite the positive correlation with age, in this sample, differences between age groups and their visit frequency were also not significant (Qui-square; p = 0.453).
A total of 289 drugs were analysed. The median number of drugs used per patient was 5.0 [IQR: 3.0 – 7.0]. As the variable “number of drugs” had no normal distribution (Shapiro-Wilk; p <0.0001), a new variable was categorized considering the median and using the numerical category most often used in polypharmacy literature - 5 or more drugs (26). With this new variable, it was found that 43.6% of patients used 4 drugs or less, and 56.4% used 5 or more drugs. No differences were identified in the number of drugs between genders (Mann-Whitney; p = 0.725) (Table 2). Age had a positive correlation with the patient's total number of medications (Spearman’s coefficient = 0.345, p = 0.010).
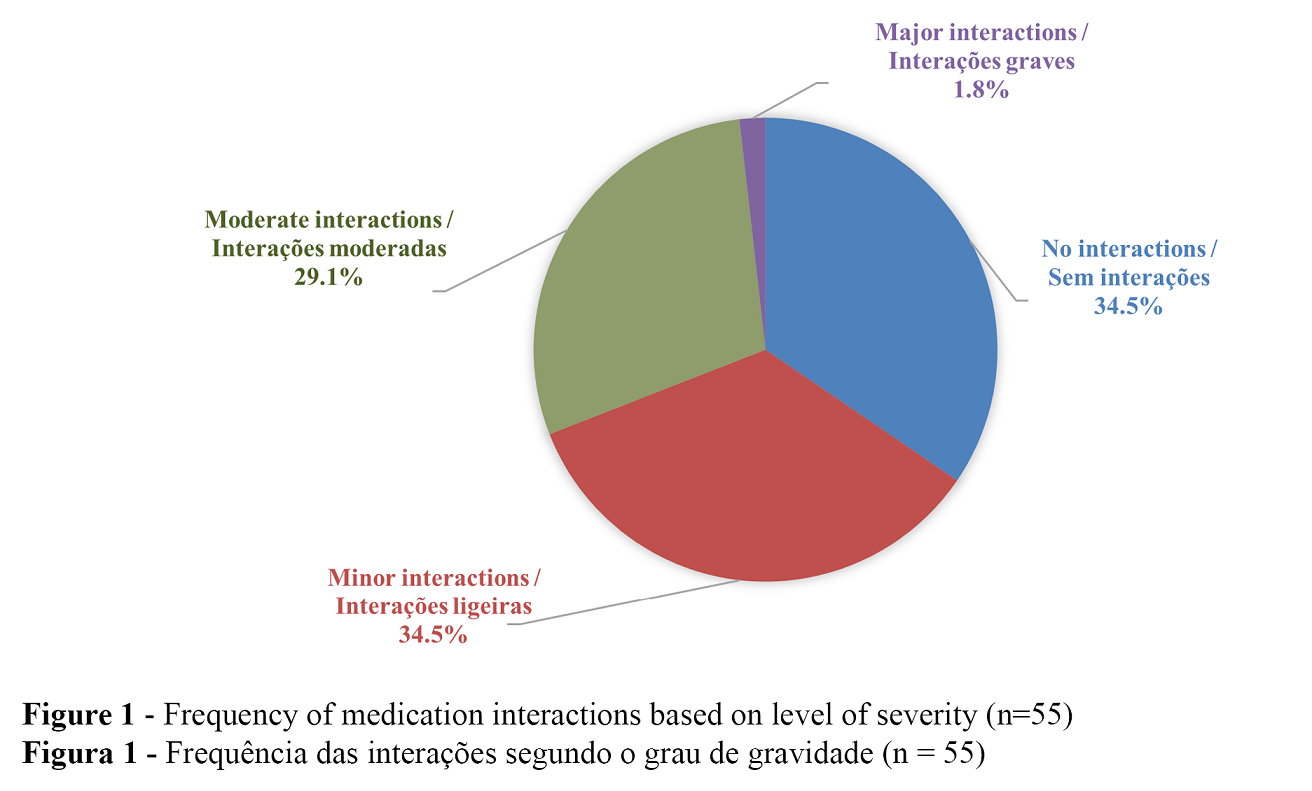
Type 1 medication review showed that 87.3% of patients in the studied sample had at least one medical condition with measurable biomarkers, such as hypertension, diabetes, dyslipidaemia, or asthma. All participants over 65 years of age had one of these medical conditions (p = 0.001), but having measurable biomarkers was not associated with the number of medications taken (Mann-Whitney; p = 0.279). Concerning 2015 Beers criteria, 18.2% of the sample population was found to take inappropriate drugs. Gender differences in Beers criteria were not significant (p = 0.148), yet a positive correlation between the number of drugs and the Beers criteria was found (Spearman coefficient 0.322; p = 0.017). When analysing drug interactions, 30.9% were found to have moderate or major interaction potential. Figure 1 shows the distribution of degrees of severity of drug interactions. No differences in the degree of severity of interaction were observed between the age groups (p=0.388), between genders (p=0.681), or patients with or without measurable biomarkers (p = 0.841). However, as with the Beers criteria, a positive correlation was found between the number of drugs and the severity degree of interactions (Spearman coefficient 0.671; p <0.001). The more medications one uses, the greater the likelihood of serious interactions. Another positive correlation was found between the severity of the interactions and Beers criteria (Spearman's coefficient = 0.343, p = 0.010), which means that patients with Inappropriate Beers criteria had also a higher likelihood of using medications with moderate or major interactions.
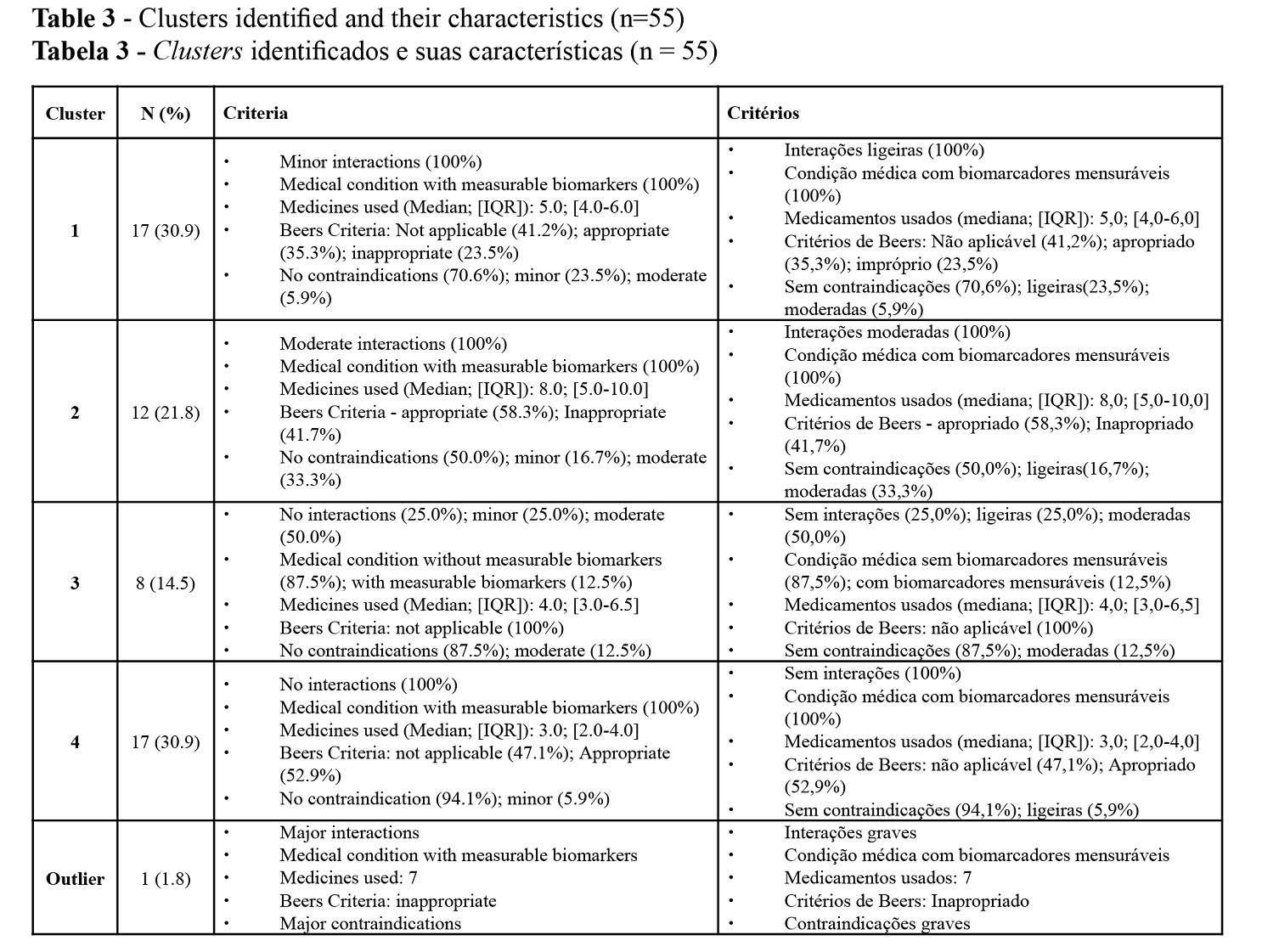
Multicollinearity was also assessed. The highest Variance inflation factor (VIF) was 2.58 for age and number of medications. The literature considers this value to be an acceptable value, indicative of low multicollinearity. After the descriptive analysis, to uncover possible patient clusters in this sample we performed a two-step cluster analysis. Considering the statistical analysis, the variables included in the model for the two-step cluster analysis were severity degree of interactions, severity degree of contraindications, Beers criteria, number of drugs used and medical condition with measurable biomarkers.
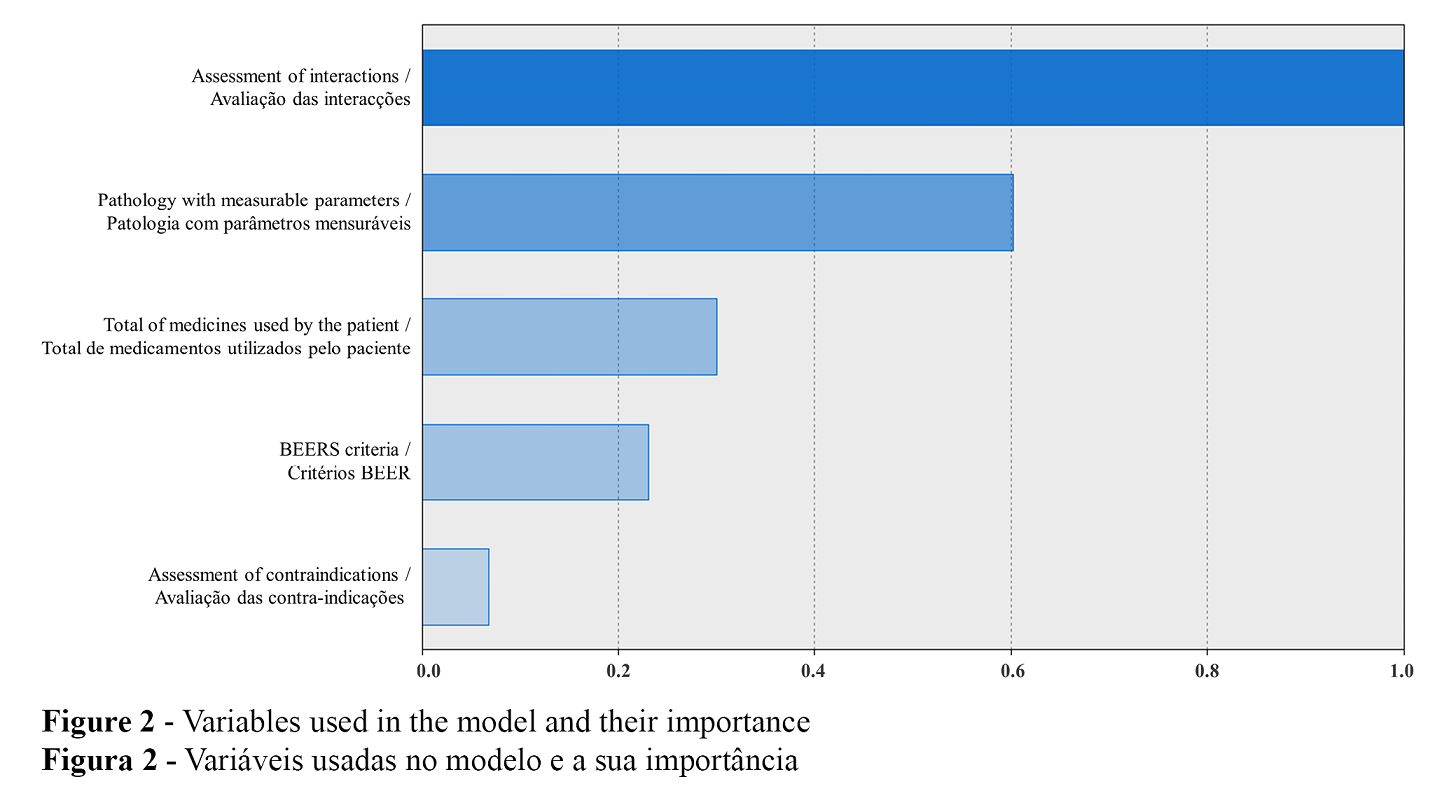
Four clusters and one outlier patient were identified, with the characteristics presented in Table 3. Figure 2 details the importance of the variables in the model. These clusters allowed to identify the proportion of patients who could benefit from different pharmaceutical interventions and their degree of urgency or priority.
Discussion
In this study, a type 1 medication review was performed using a sample of patients’ EHRs within a community pharmacy database. This review enabled the identification of patient clusters. These clusters led us to propose different pharmaceutical interventions suitable to the perceived associated risk. For instance, two clusters (2 and 3), representing 36.3% of patients, could benefit from a type 2A review, while another cluster (cluster 4) comprising 30.9% of patients would only need to measure their biomarkers to assess the effectiveness of the therapy. The medication review also identified one high risk patient requiring an urgent type 2A medication review. The results showed that all of the patients in this sample could benefit from professional intervention beyond the basic medication dispensing service. Our analysis showed that while 69% could benefit from more advanced interventions, simple interventions already integrated in day-to-day pharmacy practice would be suitable for 31% of patients. These services are low-cost, and have the potential to yield a high degree of patient engagement and satisfaction, critical for a successful longitudinal provision of services (27). The high frequency of visits to the pharmacy (1 to 2 times a month), especially in patients who use a higher number of chronic medications, gives community pharmacists the opportunity to provide such professional services without scheduling additional visits. This high frequency is common in the community setting and has been reported in other studies (28–30). Moreover, this high degree of patient-pharmacist interaction represents opportunities to provide additional care. Consistent registration of biomarkers in the patient's electronic record would allow the assessment of the effectiveness of therapies, which our study could not characterize due to the absence of biomarker records. The quality of information held within patient medication records affects the efficiency of new service provided. With more accurate and complete records, the more patient-centered approaches can be taken, with reduced duplication of effort (31).
The main causes of DRP assessed were related to safety – drug interactions, contraindications, and drug appropriateness. DRP identification is one of the first steps in therapeutic optimization, followed by its classification and resolution by the pharmacist or prescriber. As the four cluster model shows, the most important factor in cluster creation was drug interactions. This finding led us to consider the outlier patient as a high risk patient that was undetected at the time of dispensition of medication but in urget need of a type 2A medication review. The use of Sifarma™ software in the medication review proved to be useful, as it adds a degree of severity to the causes of DRPs, helping to prioritize patients and define the professional intervention of the pharmacists most suited to them.
Among the barriers to implementation of new pharmacy services thoroughly identified in the literature, lack of time is constantly cited as one of the most important (11,12). To overcome this barrier, Information Technologies would be a very welcome tool (13). Hence, the need to use an algorithm to identify patients for advanced interventions, integrating a new customer relationship service in the pharmacy workflow without massive disruption, is evident. Having an automated algorithm able to find such patients that would complement current information systems would be an obvious benefit for any pharmacy and pharmacist. In order to help pharmacists in the provision of medication reviews without increasing workload, full automation of type 1 medication review is desirable. This non-clinical review addresses simple issues related to the medication to highlight more complex issues requiring other types of reviews or services. A computerised system based on an algorithm might support a less time-consuming, continuous, and reproducible type 1 medication review (18). The automation of a type 1 medication review involves screening the pharmacy database (from Sifarma™), identifying groups of patients based on predefined criteria, and the suggestion of a professional service. Nevertheless, the full automation of all types of medication reviews cannot be foreseen. No automation replaces pharmacist's assessment. However, accessibility to well-kept electronic patient records is essential for the use of queries and a starting point for criteria-based algorithms (18). Machine Learning, and its ability to use algorithms capturing complex, nonlinear relationships in data, could then make this goal more feasible and efficient (13). We consider that this study was a step in this direction. Future studies on this topic should focus on testing an automated algorithm based on the criteria here identified, assessing its validity, pertinence, and impact on the pharmacy practice. Its use in pharmacy daily practice will enable continuous improvements and more accurate outcome measurement.
This study presents some limitations that must be acknowledged. In spite of the low number of patients in the sample, we consider that it is relevant to show the potential use of the information and data available in the pharmacy's electronic records, since this information is collected consistently over time but remains untapped. However, the low number of patients in the sample hinders the extrapolation of the medication review results to the general population. The frequency and degree of contraindications and interactions are not generalizable. Moreover, most of the drugs targeted for review were solid oral dosage forms, but it is also necessary to pay attention to non-solid dosage forms. This may have an impact in determining the severity of interactions, since the software only identifies the drug interaction, leaving the assessment of its importance to the pharmacist. In addition, the identification of potentially inappropriate medications would have been more complete if there had been access to the respective patient´s diagnosis, as has been noted in a previous study (25). Nevertheless, Lavrador et al. (25) found that although restricted access to patients’ diagnoses may limit the judgement of Beers PIM-qualifying criteria, this limitation had no effect on the number of PIM identified. This finding confirms that the identification of PIM even without diagnostic information is feasible and should be performed.
The definition of clusters using two-step cluster analyses can also be questioned. Although this method is essentially descriptive and therefore adapted to the exploratory analysis here reported, part of the assumptions for its validity, namely the normal distribution of continuous variables, were not present. However, as the variables included were independent from each other, the categorical variables had a multinomial distribution, and there was a low multicollinearity degree assessed by the VIF, it is possible that, even if all the assumptions are not fully met, the resulting clusters are still valid (32). As the objective was to identify clusters in this sample, the results presented here can only be applied to the pharmacy where the study took place. The rational for attributing pharmaceutical services to each cluster is also open for debate. Our choice was determined by our view of the medication review process, where type 1 medication review implies the identification of DRPs and making recommendations. The screening of the pharmacy database, the criteria that allow signalling the patients, an output that identifies the patient, the reasons for the signalling and the suggestion / recommendation of a professional service are the necessary cornerstones of a future intervention. Based on this output, the pharmacist must assess the situation, schedule the service and provide it, registering new information that will then feed into the algorithm, providing the basis for a “roundabout” strategy for customer loyalty as well as a contribution to the sustainability of the pharmacy. Future medication reviews using an automated algorithm in a larger set of pharmacies with a higher number of patients should validate the identified clusters.
Conclusions
Current pharmacy practice generates a wealth of information regarding patients that is underused. To provide more and better professional services, community pharmacists need tools and systems that can analyse this information, processing data easily, quickly, and continuously.
The type 1 medication review conducted in the patients’ electronic records of a pharmacy, using the Sifarma™ system, alerted to drug interactions and contraindications that, combined with the Beers criteria, allowed the identification of potential DRPs. The statistical analysis then allowed the grouping of patients into clusters, enabling their prioritization and subsequent suggestions of pharmaceutical interventions according to their health needs.
With current IT technology, we were able to identify patients who could benefit from professional pharmaceutical services, select the most appropriate intervention, and suggest their scheduling according to the frequency of the patients’ visits to the pharmacy. Further studies will include the testing of a new IT tool to allow continuous screening of the pharmacy database to identify patients at risk who will benefit most from the professional services of the pharmacy. With an algorithm-based system and new pharmacy workflows, the ubiquitous barrier of lack of time can be overcome, enhancing the community pharmacist’s ability to contribute to the quality use of medications.
Authors Contributions Statement
LR, conceptualization and study design; MM and LL, experimental implementation; LR, MM, and JG, data analysis; LR and JG, drafting, editing and reviewing; LR, figures and graphics; JG, supervision and final writing.
Funding
This study was supported by Farmácia Central do Cacém, Lda., and funded by national funds through FCT - Foundation for Science and Technology, I.P., under the UIDB/04567/2020 and UIDP/ 04567/2020 projects. João Gregório is funded by Foundation for Science and Technology (FCT) Scientific Employment Stimulus contract with the reference number CEEC/CBIOS/EPH/2018
Acknowledgements
The authors would like to express their thanks Farmácia Central do Cacém, Lda.
Conflict of Interests
The authors declare there are no financial and/or personal relationships that could present a potential conflict of interests.
References
- Meraya, A. M., Raval, A. D., & Sambamoorthi, U. (2015).Chronic condition combinations and health care expenditures and out-of-pocket spending burden among adults, Medical Expenditure Panel Survey, 2009 and 2011. Preventing Chronic Disease, 12, E12. https://doi.org/10.5888/pcd12.140388.
- Bodenheimer, T., Wagner, E. H., & Grumbach, K. (2002). Improving primary care for patients with chronic illness. JAMA, 288(14), 1775–1779. https://doi.org/10.1001/jama.288.14.1775.
- Makowsky, M. J., Schindel, T. J., Rosenthal, M., Campbell, K., Tsuyuki, R. T., & Madill, H. M. (2009). Collaboration between pharmacists, physicians and nurse practitioners: a qualitative investigation of working relationships in the inpatient medical setting. Journal of Interprofessional Care, 23(2), 169–184. https://doi.org/10.1080/13561820802602552.
- Cranor, C. W., Bunting, B. A., & Christensen, D. B. (2003). The Asheville Project: long-term clinical and economic outcomes of a community pharmacy diabetes care program. Journal of the American Pharmaceutical Association (Washington, D.C. : 1996), 43(2), 173–184. https://doi.org/10.1331/108658003321480713.
- Hatah, E., Braund, R., Tordoff, J., & Duffull, S. B. (2014). A systematic review and meta-analysis of pharmacist-led fee-for-services medication review. British Journal of Clinical Pharmacology, 77(1), 102–115. https://doi.org/10.1111/bcp.12140.
- Huiskes, V. J., Burger, D. M., van den Ende, C. H., & van den Bemt, B. J. (2017). Effectiveness of medication review: a systematic review and meta-analysis of randomized controlled trials. BMC Family Practice, 18(1), 5. https://doi.org/10.1186/s12875-016-0577-x).
- Pharmaceutical Care Network Europe Foundation (2012). PCNE statement on medication review 2011-2012.
- Blenkinsopp, A., Bond, C., & Raynor, D. K. (2012). Medication reviews. British Journal of Clinical Pharmacology, 74(4), 573–580. https://doi.org/10.1111/j.1365-2125.2012.04331.x).
- Soares, M. A., Fernandez-Llimos, F., Cabrita, J., & Morais, J. (2011). Critérios de avaliação de prescrição de medicamentos potencialmente inapropriados: uma revisão sistemática [Tools to evaluate potentially inappropriate prescription in the elderly: a systematic review]. Acta Medica Portuguesa, 24(5), 775–784..
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel (2019). American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society, 67(4), 674–694. https://doi.org/10.1111/jgs.15767.
- Niquille, A., Lattmann, C., & Bugnon, O. (2010). Medication reviews led by community pharmacists in Switzerland: a qualitative survey to evaluate barriers and facilitators. Pharmacy Practice, 8(1), 35–42. https://doi.org/10.4321/s1886-36552010000100004.
- Coane, S., & Payne, R. (2016) Carrying out a structured medication review. Prescriber27,22–26. https://doi.org/10.1002/psb.1426
- Gregório, J., & Cavaco, A. (2021). The pharmacist’s guide to the future: Are we there yet?. Research in Social & Administrative Pharmacy : RSAP, 17(4), 795–798. https://doi.org/10.1016/j.sapharm.2020.05.029
- Baines, D., Nørgaard, L. S., Babar, Z. U., & Rossing, C. (2020). The Fourth Industrial Revolution: Will it change pharmacy practice?. Research in Social & Administrative Pharmacy : RSAP, 16(9), 1279–1281. https://doi.org/10.1016/j.sapharm.2019.04.003).
- Gulavani, S. S., & Kulkarni, R. V. (2010) Role of information technology in health care. Proceedings of the 4th National Conference; INDIACom-2010.
- Jackson, S., & Peterson, G. (2019). My Health Record: a community pharmacy perspective. Australian Prescriber, 42(2), 46–47. https://doi.org/10.18773/austprescr.2019.009
- van Boven, J. F., Stuurman-Bieze, A. G., Hiddink, E. G., Postma, M. J., & Vegter, S. (2014). Medication monitoring and optimization: a targeted pharmacist program for effective and cost-effective improvement of chronic therapy adherence. Journal of Managed Care & Specialty Pharmacy, 20(8), 786–792. https://doi.org/10.18553/jmcp.2014.20.8.786.
- de Wit, H. A., Mestres Gonzalvo, C., Hurkens, K. P., Mulder, W. J., Janknegt, R., Verhey, F. R., Schols, J. M., & van der Kuy, P. H. (2013). Development of a computer system to support medication reviews in nursing homes. International Journal of Clinical Pharmacy, 35(5), 668–672. https://doi.org/10.1007/s11096-013-9827-3.
- Roten, I., Marty, S., & Beney, J. (2010). Electronic screening of medical records to detect inpatients at risk of drug-related problems. Pharmacy World & Science : PWS, 32(1), 103–107. https://doi.org/10.1007/s11096-009-9352-6.
- Poudel, A., Ballokova, A., Hubbard, R. E., Gray, L. C., Mitchell, C. A., Nissen, L. M., & Scott, I. A. (2016). Algorithm of medication review in frail older people: Focus on minimizing the use of high-risk medications. Geriatrics & Gerontology International, 16(9), 1002–1013. https://doi.org/10.1111/ggi.12589).
- Thiruchelvam, K., Wong, P. S., Kairuz, T., Babar, Z. U., & Hasan, S. S. (2018). Consolidated Medication Review Algorithm to Improve Medications Use in Older Adults: Components, Scoring Scheme, and Implementation. Journal of the American Medical Directors Association, 19(8), 717–718. https://doi.org/10.1016/j.jamda.2018.03.007).
- Grupo de Investigación en Atención Farmacéutica (CTS131). Universidad de Granada; Grupo de Investigación en Farmacología Aplicada y Farmacoterapia (CTS259). Universidad de Sevilla; Grupo de Investigación en Farmacología (CTS164). Universidad de Granada (2002) Segundo consenso de Granada sobre problemas relacionados con medicamentos. Ars Pharmaceutica, 43(3-4): 179-187. http://hdl.handle.net/10481/28237
- ANF - Sifarma Software. (2019) Manual da Compenente Profissional - Sifarma 2000 Software.
- By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel (2015). American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society, 63(11), 2227–2246.
- Lavrador, M., Silva, A. A., Cabral, A. C., Caramona, M. M., Fernandez-Llimos, F., Figueiredo, I. V., & Castel-Branco, M. M. (2019). Consequences of ignoring patient diagnoses when using the 2015 Updated Beers Criteria. International Journal of Clinical Pharmacy, 41(3), 751–756. https://doi.org/10.1007/s11096-019-00828-0.
- Masnoon, N., Shakib, S., Kalisch-Ellett, L., & Caughey, G. E. (2017). What is polypharmacy? A systematic review of definitions. BMC Geriatrics, 17(1), 230. https://doi.org/10.1186/s12877-017-0621-2.
- Gregório, J., Russo, G., & Lapão, L. V. (2016). Pharmaceutical services cost analysis using time-driven activity-based costing: A contribution to improve community pharmacies’ management. Research in Social & Administrative Pharmacy : RSAP, 12(3), 475–485. https://doi.org/10.1016/j.sapharm.2015.08.004.
- Irish Pharmacy Union (2018). Vision for community pharmacy in Ireland. https://ipu.ie/wp-content/uploads/2018/04/PwC_IPU-report.pdf.
- Boardman, H., Lewis, M., Croft, P., Trinder, P., & Rajaratnam, G. (2005). Use of community pharmacies: a population-based survey. Journal of Public Health (Oxford, England), 27(3), 254–262. https://doi.org/10.1093/pubmed/fdi032.
- Gregório, J., Cavaco, A. M., & Lapão, L. V. (2017). How to best manage time interaction with patients? Community pharmacist workload and service provision analysis. Research in Social & Administrative Pharmacy : RSAP, 13(1), 133–147. https://doi.org/10.1016/j.sapharm.2016.02.008.
- Wright, D. J., & Twigg, M. J. (2016). Community pharmacy: an untapped patient data resource. Integrated Pharmacy Research & Practice, 5, 19–25. https://doi.org/10.2147/IPRP.S83261
- IBM. Clustering Principles. (2020) IBM Knowledge Center[www.ibm.com/support/knowledgecenter/pt/SSLVMB_24.0.0/spss/tutorials/twostep_methodology.html|
