![]()
Biomedical Sciences, Biomed Biopharm Res., 2021; 18(1):25-37
doi: 10.19277/bbr.18.1.259; download pdf version [+] here
Antioxidant activities and antidiabetic potential of extract of fruits from the Myrtaceae family: inhibitory effects on α-amylase and α-glucosidase activities
Simone Muniz Pacheco 1, Mauricio Seifert 2, Rafael de Almeida Schiavon 3, Maiara Sandrielly Pereira Soares 1, Rejane Giacomelli Tavares 1,4*, Mauricio Seifert 2, Leonardo Nora 2
1Centro de Ciências Químicas, Farmacêuticas e de Alimentos- CCQFA- Universidade Federal de Pelotas, Campus Universitário, S / N, Capão do Leão - RS, 96160-000, Brasil; 2Departamento de Ciências Agroindustrial e Tecnologia- Faculdade de Agronomia Eliseu Maciel- Universidade Federal de Pelotas, Campus Universitário, S / N, Capão do Leão - RS, 96160-000, Brasil; 3Centro de Ciências da Agricultura, Departamento de Engenharia Agrícola, Universidade Estadual de Maringá, Av Colombo, 5790, 87020-900, Maringá, PR, Brasil; 4CBIOS/ ECTS-Universidade Lusófona, Av Campo Grande, 376, 1749-024, Lisboa, Portugal
*corresponding author:
Abstract
There is a great diversity of plants which are grown in the Atlantic Forest region of Brazil that produce small, colorful, edible fruit that are used in empiric mode to treat several diseases, such as diabetes, as fruits are a rich source of dietary phenolic antioxidants. In this study, we investigated the inhibitory activity of methanolic fruit extracts from the Myrtaceae family - Psidium cattleianum (araçá), Syzygium cumini (jambolão), Campomanesia xanthocarpa (guabiroba), Eugenia uniflora (pitanga) and Eugenia pyriformis (uvaia) - against α-amylase and intestinal α-glucosidase (maltose and sucrose). The antioxidant activities were evaluated using two different in vitro assays: the 2,2’-azinobis(3-ethylbenzthiazoline-6-sulphonate) (ABTS) test and the 2,2’-diphenyl-1-picrylhydrazyl (DPPH) test. The extracts of P. cattleianum, S. cumini, E. pyriformis inhibited α-amylase activity between 13% and 60% (p<0.05). The extracts of P. cattleianum also inhibited α-glucosidase activity with either maltose or sucrose as substrate between 15% and 61% (p<0.05). Additionally, these fruits are rich in phenolic compounds with antioxidant activities.
Keywords: Myrtaceae, diabetes, phenolics, α-glucosidase, α-amylase
Received: 11/04/2021; Accepted: 04/06/2021
Introduction
Chronic hyperglycemia due to carbohydrate metabolism alterations is the main characteristic of type 2 diabetes mellitus (T2DM). This disease frequently does not present many symptoms and thus can remain undiagnosed and untreated for many years, leading to micro- and macrovascular damage to the eyes, kidneys, heart, and nerves (1). Recommendations to prevent or to delay the complications of this disease include a healthy diet, regular physical exercise, and corporal weight control (lifestyle change) (2).
Several drugs are indicated to treat T2DM, including glucosidase inhibitors such as acarbose, miglitol, and voglibose (3). These drugs act through the interruption or slowing of the digestion of dietary starch in order to decrease the rate of blood sugar absorption in the small intestine by the inhibition of the activity of α-amylase and α-glucosidase enzymes (4,5). Although blood glucose is reduced, the glucosidase inhibitors may generate some unexpected adverse effects, such as diarrhea, abdominal pain, flatulence (3), and weight gain (6).
Consumption of fruits rich in phenolic compounds is associated with health benefits, and there is evidence that some fruit extracts inhibit enzymes involved in carbohydrate metabolism. Thus, fruit extracts have been shown to be a potential source of antihyperglycemic compounds, capable of slowing down glucose absorption with minimum side effects. The Myrtaceae family is one of the world’s leading commercial fruit tree families with great potential to be explored economically, with fruits presenting excellent nutritional value. Among the various genera belonging to this family, leaf and fruits extracts from Psidium cattleianum (araçá), Syzygium cumini (jambolão), Campomanesia xanthocarpa (guabiroba), Eugenia uniflora (pitanga), and Eugenia pyriformis (uvaia) have been investigated in the treatment of diabetes. The fruits are considered sources of phytochemicals such as phenolic compounds, carotenoids, and volatile compounds. Many of these phytochemicals have the capability to act in controlling oxidative stress and protein glycation due to their potential to decrease hyperglycemia and hyperlipidemia by the inhibition of the catalysis of digestive enzymes. They are related to the prevention and management of several chronic and degenerative diseases, including cancer, cardiovascular diseases, obesity, amnesia among other disorders (5,7,8). These genera are common in the Atlantic Forest region in Brazil and are widely used in folk medicine to treat hyperglycemia, but without scientific evidence to support the effectiveness of treatment. Therefore, the aim of this study was to characterize fruits from plants of Myrtaceae family (Psidium cattleianum, Campomanesia xanthocarpa, Eugenia pyriformis, Eugenia uniflora and Syzygium cumini) that grow in the Southern region of the Brazilian Atlantic Forest through determination of inhibitory potential of α-amylase and α-glucosidase, their total phenolic and total flavonoid content, and their antioxidant activity.
Materials and Methods
Analytical reagents and chemicals
Reagents, solvents, and enzymes were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Acarbose was purchased from Bayer (Glucobay® 100) and the glucose assay kit (Glicose GOD) was purchased from Labtest® (Minas Gerais, Brazil).
Plant material
The fully ripe fruit from Psidium cattleianum (red fruit, accesses 44 and 87, and yellow fruit, access ‘bicudo’), Campomanesia xanthocarpa, Eugenia pyriformis (accesses 3, 4, 11, and 15) and Eugenia uniflora (access 156) were harvested from the experimental field at ‘Embrapa Clima Temperado’, Research Station, in Pelotas, RS, Brazil, (31° 42′ S, 52° 24′ W, Altitude: 57 m). Each access corresponds to a different germoplasm, identified and registered in the Active Germplasm Bank. The fully ripe fruit from Syzygium cumini was harvested from the experimental field at ‘Universidade Federal de Pelotas’, Capão do Leão, RS, Brazil. Only commonly edible parts were used for the analyses – whole fruit for P. cattleianum and C. xanthocarpa; peel and pulp for E. pyriformis, E. uniflora and S. cumini. Fruits were selected, processed, freeze-dried and stored at - 20°C.
Preparation of fruit extracts
Extract preparation was based on the method of Alothman et al. (2009), with modifications. Frozen fruits (200 g) were ground to a fine powder in a ball mill (Marcone MA350, São Paulo, Brazil) with liquid nitrogen and mixed (1:3, weight (w)/volume (v) ratio) with an extraction solvent (methanol:water, 80:20, v/v ratio). Samples were incubated for 3 hours in a water bath with stirring and heating (40°C). Extracts were filtered, dried in a rotatory evaporator at 40°C (La Borota 4000 Heidolph, Schawabach, Germany) and freeze-dried (Enterprise Terroni, São Carlos (SP), Brazil). Extracts were prepared in triplicate and dissolved in methanol/water (1:3) to a concentration of 5,0 g.ml -1 and stored at - 20°C until analysis.
Determination of total phenolic content
Total phenolics were determined by using the Folin-Ciocalteu method described by Swain and Hillis (10). Results were expressed as milligram of gallic acid equivalents per 100 g fresh weight (mg of GAE/100 g fw).
Determination of total flavonoids content
The total flavonoid content was measured according to the method described by Zhishen et al. (11). Results were expressed as milligram catechin equivalents per 100 g fresh weight (mg of CE/100 g fw).
DPPH and ABTS radical-scavenging assay
Antioxidant activity was determined using the DPPH radical scavenging method according to Brand-Williams et al. (12). The ABTS method was performed according to Re et al. (13). Results were expressed as a percentage of inhibition of DPPH or ABTS radical, respectively.
α-amylase inhibition assay
Inhibitory activity of α-amylase was determined according to Yu et al. (14) with modifications. The tests were performed using α-amylase from Bacillus licheniformis (40 units/mL), aqueous extracts at different concentrations (0.1; 0.25; 0.5; 1.0; 2.5; 5.0; 10.0 mg/mL) and a solution of soluble starch (1%). Acarbose was used as positive control (5 mM) (15). The inhibition (%) was calculated by using the following formula:
![]()
α-glucosidase inhibition assay
Intestinal α-glucosidase inhibitory activity was based on the method of Adisakwattana et al. (16) with modifications. Intestinal acetone powder from rat (Sigma-Aldrich catalog nº I1630) were used for the enzyme solution and maltose (86 mM) or sucrose (400 mM) solutions as the substrate. Fruits extracts were used in different concentratios (1; 2.5; 5.0; 10.0 mg/mL) in aqueous solution. Acarbose was used as a positive control (5 mM) (15). The inhibition (%) was calculated by using the following formula:
![]()
Statistical analysis
The results were expressed as mean values and standard error (SE). Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s Test with p<0.05. The statistical program used was GraphPad Prism® version 5.0 (San Diego, California, USA). All analyses were performed in triplicate.
Results and Discussion
Phenolic content and antioxidant capacity
The total content of phenolic compounds and flavonoids of the fruits analyzed in this study are shown in Table 1. The total phenolic compound content ranged from 181.48 to 541.15 mg of GAE/100 g fw. S. cumini had the highest phenolic compound content (541,15 mg of GAE/100 g fw) followed by C. xanthocarpa (495,86 mg of GAE/100 g fw) and P. cattleianum (all accesses) (range 435,81 to 445,64 mg of GAE/100 g fw). Gajera et al. (17) evaluated the phenolic content in methanolic extract of S. cumini and describe results similar to those in our study. According the authors, seven phenolic compounds (gallic acid, catechin, chlorogenic acid, caffeic acid, ferulic acid, ellagic acid and quercetin) were identified in higher concentrations in seeds and seeds parts. In the P. cattleianum, we observed similar quantitative results as reported by Pereira et al. (18). However, discrepant higher values (632.56 to 581.02 mg of GAE/100 g fw) were reported by Medina et al. (19), who evaluated the composition of pulp in aqueous extracts. These differences can be due to several factors, such as humidity, soil type, climate during the development phase, stage of maturation (20), genetic differences, and postharvest storage conditions (21). The part(s) of the fruit utilized and the method of extraction can also contribute to these differences (18).
In order to determine the antioxidant activity of the extracts, two distinct methods were used (DPPH and ABTS) (Table 1). The highest percentage of inhibition of DPPH radicals were observed in C. xanthocarpa (95.13%), S. cumini (94.53%) and P. cattleianum (all accesses) (93.95% to 95.62%), which correlated with the highest levels of phenolic compounds detected. Similar results were observed in the inhibition of ABTS radicals. Total phenolic content is positively correlated with antioxidant activity. In fact, major phenolic compounds related to fruits from Myrtaceae family, such as gallic acid, catechin, ellagic and ferulic acids are strongly linked to antidiabetic and free radical scavenging activity (17). Chelation, inhibition of lipid peroxidation, anti-inflammatory, and antiproliferative properties are also linked to phenolic compounds (18). Other authors have also described the antioxidant property of methanolic extract of S. cumini in leaves, fruit peel, and leaf gall, corroborating with our results (22).
Inhibitory activities against α-amylase and α-glucosidase
Currently available drugs for the control of hyperglycemia are effective, however, the search for alternatives that will have fewer side effects is of great interest. Therefore, this study assessed fruit extracts for their inhibitory potential in the activity of α-amylase and α-glucosidase which are directly related to glucose absorption. Extracts of plants that are rich in phenolic compounds have been described as inhibiting both enzymes (5). In our study, P. cattleianum access 44 (2.5 mg/mL), S. cumini (0.1, 0.25, 0.5 and 1.0 mg/mL) and E. pyriformis access 11 (0.1, 0.25, 0.5 and 1,0 mg/mL) and access 15 (0.1, 0.25 and 0.5 mg/mL) extracts significantly inhibited (p<0.05) α-amylase activity in vitro (Figure 1). The extract of S. cumini demonstrated the highest percentage of α-amylase activity inhibition (60.50 ± 1.37%) at lower concentrations (0.1 mg/mL). In an in vivo study, rats fed with ethanolic extract of S. cumini seed and pulp were observed to have an ameliorated / lower production of insulin and a better blood sugar balance (17). This effect was attributed to ellagic acid and its ability to stop the conversion of starch into sugar when glucose level increases in blood (17,23). The α-amylase inhibition is also attributed to catechins. These are able to bind to the active site side chains, resulting in a complex that prevents the substrate from binding (noncompetitive inhibition), and variations in catechin structure can affect their power of inhibition. The addition of a galloyl group, for example, creates a powerful inhibitor inhibition (24). Similarly to our results, Poonguran et al. (25) also demonstrate a significantly higher inhibitory effects on α-amylase using methanol and water extracts of S. cumini leaves, rich in thetriterpenoids ursolic acid and oleanolic acid.
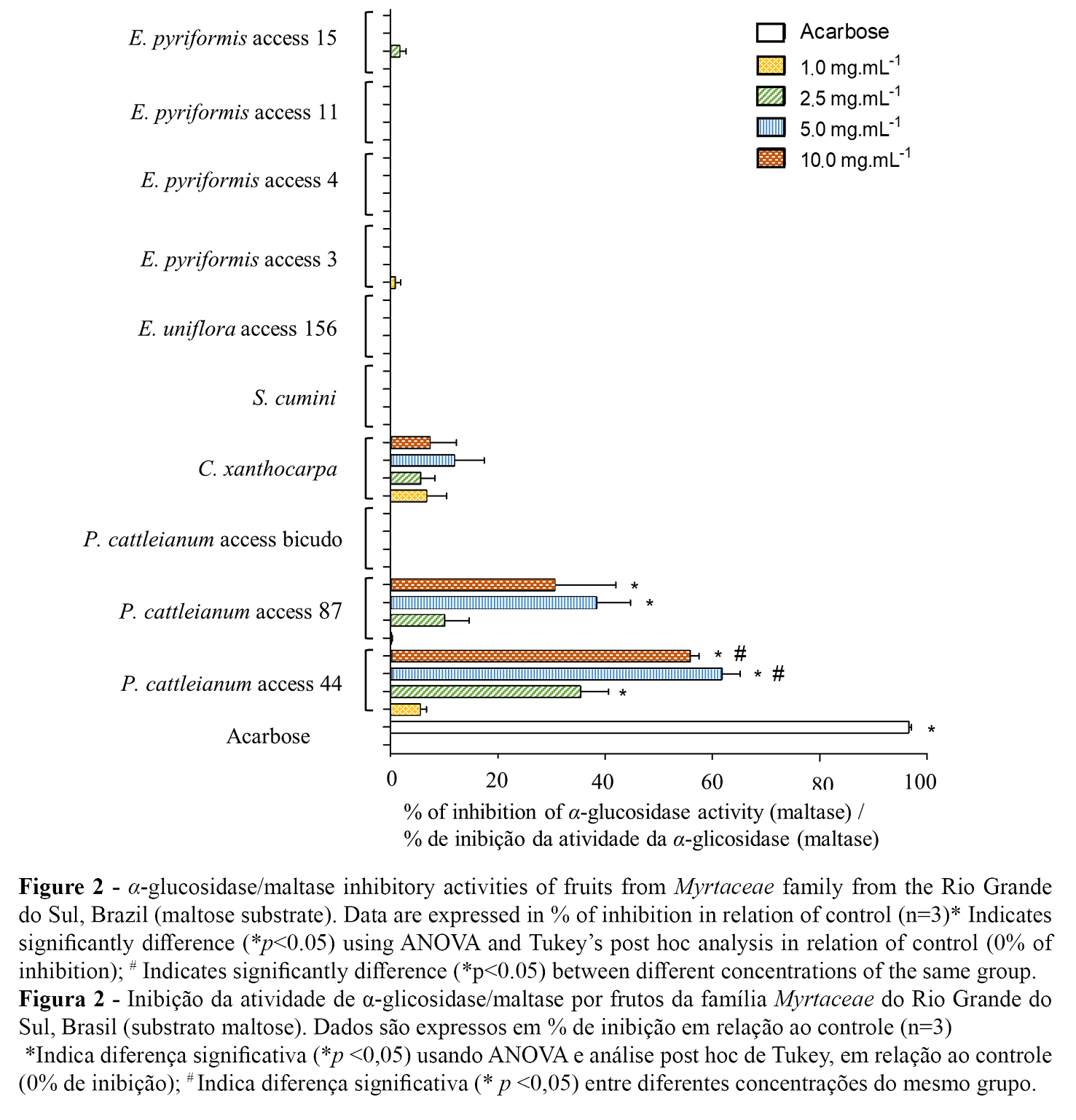
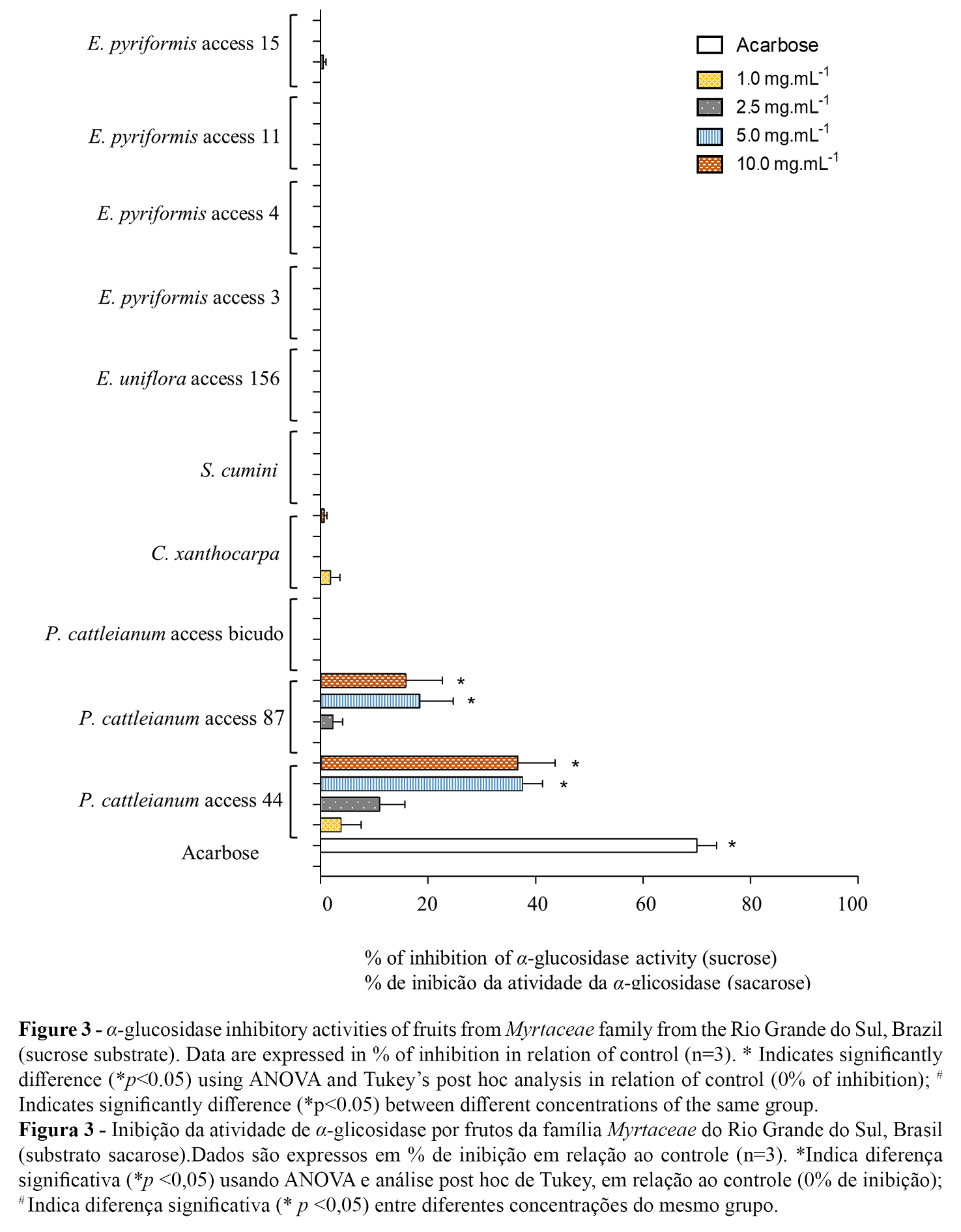
Phenolic compounds were found to be effective inhibitors of intestinal α-glucosidase/maltase activity. Phenolic compounds positively interact with enzymes by changing their biocatalytic action. The carboxyl and hydroxyl groups of phenolic acids bind to the starch through hydrogen bonds, chelation or covalent bonds, forming bridges or cross-links (23). α-Glucosidase activity was evaluated using maltose and sucrose substrates. P. cattleianum access 44 (2.5, 5.0 and 10.0 mg/ml) and access 87 (5.0 and 10.0 mg/ml) were capable of significantly inhibiting maltase activity (p<0.05) (Figure 2). When we used sucrose as substrate, P. cattleianum access 44 (5.0 and 10.0 mg/ml) and access 87 (5.0 and 10.0 mg/ml) were able to inhibit α-glucosidase activity (p<0.05) (Figure 3). Interestingly, the inhibitory effects were observed in the presence of the highest flavonoids contents. In a study with red fruits of P. cattleianum, several anthocyanins (cyanidin-3-glucoside, malvidin-3-glucoside, and cyanidin chloride) were identified as majority compounds (26). Flavonoids myricetin and quercetin, found in Hovenia Dulcis Thunb, have been reported to reversibly inhibit α-glucosidase ins a noncompetitive manner (8). It should also be noted that enzymes of different origin showed different results. When assessing inhibition of yeast α-glucosidase, cyanidin (99%), myricetin (94%) and genistein (93%) were the main inhibitors. When using α-glucosidase from rat small bowel, the main inhibitors were epigallocatechin gallate (32%), myricetin (29%), and quercetin (28%) (27). Moreover, other authors have reported the α-glucosidase inhibitory activity might be due to the present of ellagitannins, such as punicalin and punicalagin (28).
Extracts from yellow P. cattleianum access ‘bicudo’, E. uniflora, and E. pyriformis accesses 3 and 4 did not significantly inhibit α-amylase or α-glucosidase activity in our study. However the concentration of total content of phenolic compounds and flavonoids reported are similiar in red and yellow fruits of P. cattleianum, previous studies demonstrate that yellow fruits are rich in carotenoids (18) and red fruits has high content of anthocyanins (20), that may contribute to explain specific biological activities observed in our study. Pinto et al. (21) and Podsędek et al. (4) report that inhibition of the activity of both enzymes seems not to depend on the total phenolic compound content but the characteristics of the individual compounds such as concentration, structure, and interaction between them. These characteristics can contribute to the stability, solubility, and binding ability of these compounds to target enzymes (4).
Conclusion
Extracts of P. cattleianum (accesses 44 and 87), S. cumini and E. pyriformis (accesses 11 and 15) evaluated in this study were effective in inhibiting the activity of α-amylase and/or α-glucosidase in vitro, correlated with high content of phenolic compounds and antioxidant capacity. Considering the evidence which suggests that phenolic compounds are relevant in the prevention or treatment of chronic diseases, including T2DM, increasing knowledge on the biological activities of native fruits rich in this compounds could be useful for developing functional foods focus on health benefits.
Authors Contributions Statement
SMN, MS, and MSP prepared the extracts and performed enzymatic assays. RAS performed phytochemical analysis, RGT and LN performed the conceptualization and study design, SMN wrote the manuscript, RGT ensured statistical analysis, supervision, and final writing /correction of the manuscript.
Funding
This study was supported by CNPq.
Acknowledgements
We are grateful to Rodrigo Cezar Franzon and to the Embrapa Clima Temperado for the supply of fruits.
Conflict of Interests
The authors declare there are no financial and/or personal relationships that could present a potential conflict of interests.
References
- Broholm, S.L., Gramsbergen, S.M., Nyberg, T., Jäger, A.K., Staerk, D. (2019) Potential of Sorbus berry extracts for management of type 2 diabetes: Metabolomics investigation of 1H NMR spectra, α-amylase and α- glucosidase inhibitory activities, and in vivo anti-hyperglycaemic activity of S. norvegica. Journal of Ethnopharmacology, 242, 112061. https://doi.org/10.1016/j.jep.2019.112061
- Xiao, J. (2015) Natural polyphenols and diabetes: understanding their mechanism of action. Current Medical Chemistry,22(1): 2-3.https://doi.org/10.2174/0929867321666141012173816
- Hedrington, M.S. & Davis, S.N. (2019) Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opinion Pharmacotherapy, 20 (18): 2229-2235. https://doi.org/ 10.1080/14656566.2019.1672660
- Podsedek, A., Majewska, I., Redzynia, M., Sosnowska, D., Koziolkiewicz, M. (2014). In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. Journal of Agricultural and Food Chemistry, 62(20): 4610-4617. https://doi.org/10.1021/jf5008264
- Papoutsis, K., Zhang, J., Bowyer, M.C., Brunton, N., Gibney, E.R., Lyng, J. (2021) Fruit, vegetables, and mushrooms for the preparation of extracts with α- amylase and α-glucosidase inhibition properties: A review. Food Chemistry, 338 (2021) 128119.https://doi.org/10.1016/j.foodchem.2020.128119
- Carpéné, C., Gomez-Zorita, S., Deleruyelle, S., Carpéné, M. A. (2015) Novel strategies for preventing diabetes and obesity complications with natural polyphenols. Current Medical Chemistry, 22(1): 150-164. https://doi.org/10.2174/0929867321666140815124052
- Correia, R. T., Borges, K. C., Medeiros, M. F., Genovese, M. I. (2012) Bioactive compounds and phenolic-linked functionality of powdered tropical fruit residues. Food Science and Technology International, 18(6): 539-547. https://doi.org/10.1177/1082013211433077
- Sun, L. & Miao, M. (2019) Dietary polyphenols modulate starch digestion and glycaemic level: a review. Critical Reviews in Food Science and Nutrition, 1-15.https://doi.org/10.1080/10408398.2018.1544883
- Alothman, M., Bhat, R., Karim, A. A. (2009) Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extract with different solvents. Food Chemistry, 115(3): 785-788. https://doi.org/10.1016/j.foodchem.2008.12.005
- Swain, T., Hillis, W. E. (1959) The phenolic constituents of Prunus domestica. I. – The quantitative analysis of phenolic constituents. Journal of the Science of Food and Agriculture, 10(1): 63-68. https://doi.org/10.1002/jsfa.2740100110
- Zhishen, J., Mengcheng, T., Jianming, W. (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64(4): 555-559.
- Brand-Wiliams, W., Cuvelier, M. E., Berset, C. (1995) Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology, 28(1): 25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
- Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., Rice-Evans, C. (1999) Antioxidant activity applying improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9-10): 1231-1237. https://doi.org/10.1016/s0891-5849(98)00315-3
- Yu, Z., Yin, Y., Zhao, W., Yu, Y., Liu, B., Liu, J., Chen, F. (2011) Novel peptides derived from egg white protein inhibiting alpha-glucosidase. Food Chemistry, 129(4): 1376-1382.
- Jockovic, N., Fischer, W., Brandsch, M., Brandt, W., Dräger, B. (2013) Inhibition of human intestinal α-glucosidases by calystegines. Journal of Agricultural and Food Chemistry, 61(23): 5550-5557. https://doi.org/10.1021/jf4010737
- Adisakwattana, S., Ruengsamran, T., Kampa, P., Sompong, W. (2012). In vitro inhibitory effects of plant-based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Complementary & Alternative Medicine,12: 110. http://dx.doi.org/10.1186/1472-6882-12-110.
- Gajera, H.P., Getiya, S.N., Hirpara, D.G., Patel, S.V., Golakiya, B.A. (2017) Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cuminiL.) landraces. Journal of Food Science and Technology, 54(10): 3180-3191. https://doi.org/10.1007/s13197-017-2756-8
- Pereira, E.S., Vinholes, J., Franzon, R.C., Dalmazo, G., Vizzotto, M., Nora, L. (2018). Psidium cattleianumfruits: A review on its composition and bioactivity. Food Chemistry, 258:95-103. https://doi.org/10.1016/j.foodchem.2018.03.024
- Medina, A. L., Haas, L. I. R., Chaves, F. C., Salvador, M., Zambiazi, R. C., Silva, W. P., Nora, L., Rombaldi, C. V. (2011) Araçá (Psidium cattleianumSabine) fruit extracts with antioxidant and antimicrobial activities and antiproliferative effect on human cancer cells. Food Chemistry, 128(4): 916-922. https://doi.org/10.1016/j.foodchem.2011.03.119
- Biegelmeyer, R., Andrade, J. M. M., Aboy, A. L., Apel, M. A., Dresch, R. R., Marin, R., Raseira, M. C. B., Henriques, A. T. (2011) Comparative analysis of the chemical composition and antioxidant activity of red (Psidium cattleianum) and yellow (Psidium cattleianumvar. lucidum) strawberry guava fruit. Journal of Food Science, 76(7): 991C-996C. https://doi.org/10.1111/j.1750-3841.2011.02319.x
- Pinto, M. S., Kwon, Y., Apostolidis, E., Lajolo, F. M., Genovese, M. I., Shetty, K. (2010) Evaluation of red currants (Ribes rubrumL.), black currants (Ribes nigrumL.), red and green gooseberries (Ribes uva-crispa) for potential management of type 2 diabetes and hypertension using in vitro models. Journal of Food Biochemistry, 34(3): 639-660.
- Franco, R.R., Zabisky, L.F.R., Lima Júnior, J.P., Alves, V.H.M., Justino, A.B., Saraiva, A.L., Goulart, L.R., Espindola, F.S. (2020) Antidiabetic effects of Syzygium cumini leaves: A non-hemolytic plant with potential against process of oxidation, glycation, inflammation and digestive enzymes catalysis. Journal of Ethnopharmacology, 261: 113132. https://doi.org/10.1016/j.jep.2020.113132
- Chhikara, N., Kaur, R., Jaglan, S., Sharma, P., Gat, Y., Panghal, A. (2018) Bioactive compounds and pharmacological and food applications of Syzygium cumini– a review. Food and Function, 9: 6096-6115. https://doi.org/10.1039/c8fo00654g
- Miao, M., Jiang, H., Jiang, B., Li, Y., Cui, S. W., Zhang, T. (2014) Structure elucidation of catechins for modulation of starch digestion. LWT – Food Science and Technology, 57(1): 188-193.
- Poonguran, J., Perera, H.K.I., Jayasinghe, L., Fernando, I.T., Sivakanesan, R., Araya, H., Fujimoto, Y. (2017) Bioassay-guided fractionation and identification of α-amylase inhibitors from Syzygium cuminileaves. Pharmaceutical Biology, 55 (1): 206-211. https://doi.org/10.1080/13880209.2016.1257031
- Nora, C. D., Jablonski, A., Rios, A. O., Hertz, P. F., Jong, E. V., Flôres, S. H. (2014) The caracterization and profile of the bioactive compounds in red guava (Psidium cattleianumSabine) and guabiju (Myrcianthes pungens(O. Berg) D. Legrand). International Journal of Food Science & Technology, 49: 1842-1849. https://doi.org/10.1111/ijfs.12493
- Tadera, K., Minami, Y., Takamatsu, K., Matsuoka, T. (2006) Inhibition of α-glucosidase and α-amylase by flavonoids. Journal of Nutritional Science and Vitaminology, 52(2): 149-153. https://doi.org/10.3177/jnsv.52.149
- Vinhole, J., Reis, S.F., Lemos, G., Barbieri, R.L., Freitas, V., Franzon, R.C., Vizzotto, M. (2018) Effect of in vitro digestion on the functional properties of Psidium cattleianumSabine (araçá), Butia odorata(Barb. Rodr.) Noblick (butiá) and Eugenia uniflora L. (pitanga) fruit extracts. Food & Function, 9: 6380-6390.https://doi.org/10.1039/c8fo01329b
