| Review Article, Biomed Biopharm Res., 2023; 20(2):118-142 doi: 10.19277/bbr.20.2.321; Bilingual PDF [+]; Portuguese html [PT] |
Biopharmaceuticals: A review of their development and contribution to healthcare
Kai Bin Liew 1 ![]() ✉️ , Siew-Keah Lee 2
✉️ , Siew-Keah Lee 2 ![]() , Long Chiau Ming 3
, Long Chiau Ming 3 ![]() , A.B.M. Helal Uddin 4
, A.B.M. Helal Uddin 4 ![]() , Zaidul Islam Sarker 4
, Zaidul Islam Sarker 4 ![]() , Yik-Ling Chew 5
, Yik-Ling Chew 5 ![]() , & Phei Er Kee1
, & Phei Er Kee1 ![]()
1 - Faculty of Pharmacy, University of Cyberjaya, Persiaran Bestari, 63000 Cyberjaya, Selangor, Malaysia
2 - M. Kandiah Faculty of Medicine and Health Sciences, Universiti Tunku Abdul Rahman, Jalan Sungai Long, Bandar Sungai Long, 43000, Kajang, Selangor, Malaysia
3 -School of Medical and Life Sciences, Sunway University, Sunway City, 47500 Malaysia
4 - Faculty of Pharmacy, International Islamic University Malaysia, Bandar Indera Mahkota, Kuantan, Pahang, Malaysia
5 - Faculty of Pharmaceutical Sciences, UCSI University, Jalan Menara Gading, UCSI Heights, 56000 Cheras, Kuala Lumpur, Malaysia
Abstract
Biopharmaceuticals play a crucial role in preventing, treating, and diagnosing a diverse range of diseases across various medical disciplines. The market for biopharmaceuticals has experienced significant growth, driven by tremendous demand, with their dynamic market potential believed to surpass that of conventional counterparts. This review provides insight into the manufacturing process of biopharmaceuticals, covering both upstream and downstream processing. Various types of biopharmaceutical products, such as monoclonal antibodies, enzymes, vaccines, stem cells, human growth hormones, cytokines, nucleic acids, and carbohydrates, are explored alongside their respective clinical applications. The review also addresses the challenges encountered in the development, formulation, and utilization of these biopharmaceuticals. In essence, this review contributes valuable knowledge to the understanding of the expansive field of biopharmaceuticals.
Keywords: biopharmaceuticals, manufacturing, recombinant, therapeutics, clinical
To Cite: Liew, K. B., et al. (2023) Biopharmaceuticals: A review of their development and contribution to healthcare. Biomedical and Biopharmaceutical Research, 20(2),118-142.
Author Correspondence:
Received: 24/08/2023; Accepted: 14/12/2023
Introduction
The global increase in population, coupled with longer life expectancies and a rising prevalence of chronic diseases, especially regarding auto-immune diseases, has generated a substantial market demand for safer and more effective drugs (1). Since the approval of the first biopharmaceutical, recombinant human insulin by Escherichia coli, for therapeutic use in 1982 by the Food and Drug Administration (FDA), the utilization of biopharmaceuticals has been steadily growing (2). The global biopharmaceutical market, valued at $237.2 billion in 2018, is expected to reach $389.0 billion by 2024, with a compound annual growth rate (CAGR) of 8.59% from 2019 to 2024 (2). Biopharmaceuticals play a crucial role in enhancing healthcare, extending healthy productive longevity, and reducing the prevalence of severe diseases. Studies report that biopharmaceutical innovation contributed to about 35% of the increase in life expectancy from 1990 to 2015, leading to an improvement in average life expectancy from 46.5 years to 65.0 for individuals (3).
Biopharmaceuticals refer to pharmaceutical products produced via biotechnological processes, employing techniques like recombinant DNA technology or the hybridoma technique. The biological sources and living organisms such as bacteria, viruses, yeast, animal, and plant cells are commonly employed for the production of biopharmaceuticals (4, 5). Biopharmaceuticals exhibit higher molecular weight and a more complex structure, typically 100-1000 times larger than synthetic drugs, owing to the formation of polymeric chains (5). These products comprise polysaccharides, proteins, nucleic acids, tissues, and living cells, finding extensive applications across various medical fields.
Biopharmaceutical products are recognized for their efficacy in diagnosing, preventing, treating, and curing a broad spectrum of chronic and life-threatening diseases, including metabolic disorders and cancers (6). Their specificity to certain targets enables biopharmaceuticals to recognize and target specific sites and diseases, minimizing undesirable side effects. Additionally, biopharmaceuticals can serve as an alternative treatment for patients with poor responses to conventional treatments, with high bioavailability, increased half-life, and lower immunogenicity (6). Due to the complexity and instability of the products, as well as low intestinal absorption, oral administration is generally unsuitable for biopharmaceuticals. As there is the occurrence of substantial reduction in permeability across biological barriers such as skin, mucosal membranes and cell membranes, subcutaneous injection emerges as the preferred administration method (7, 8).
This review delves into the manufacturing process of biopharmaceutical products and explores various types of biopharmaceutical products, including monoclonal antibodies (mAbs), vaccines, enzymes, hormones, cell therapy products and cytokines, along with their clinical applications. The challenges encountered in the development and application of biopharmaceuticals are also discussed, providing a comprehensive overview of this dynamic field.
Manufacturing process
The manufacturing of biopharmaceuticals involves two complex stages, namely upstream processing and downstream processing. Figure 1 illustrates the flowchart of the biopharmaceutical manufacturing process. Upstream processing is related to the growth of microbes and the transformation of substrates into the desired biopharmaceutical products (9). Various activities, including cell line selection, culture medium composition, optimization of growth parameters and process, are performed to achieve optimal cell growth and biopharmaceutical productions (10). The fermentation process occurs under controlled conditions in a large-scale bioreactor system, where measures such as sterilization of materials and equipment, pH regulation, temperature control and oxygen supply are crucial to minimize the risk of contamination by other microorganisms (11).
| Figure 1. Flowchart of biopharmaceutical manufacturing process |
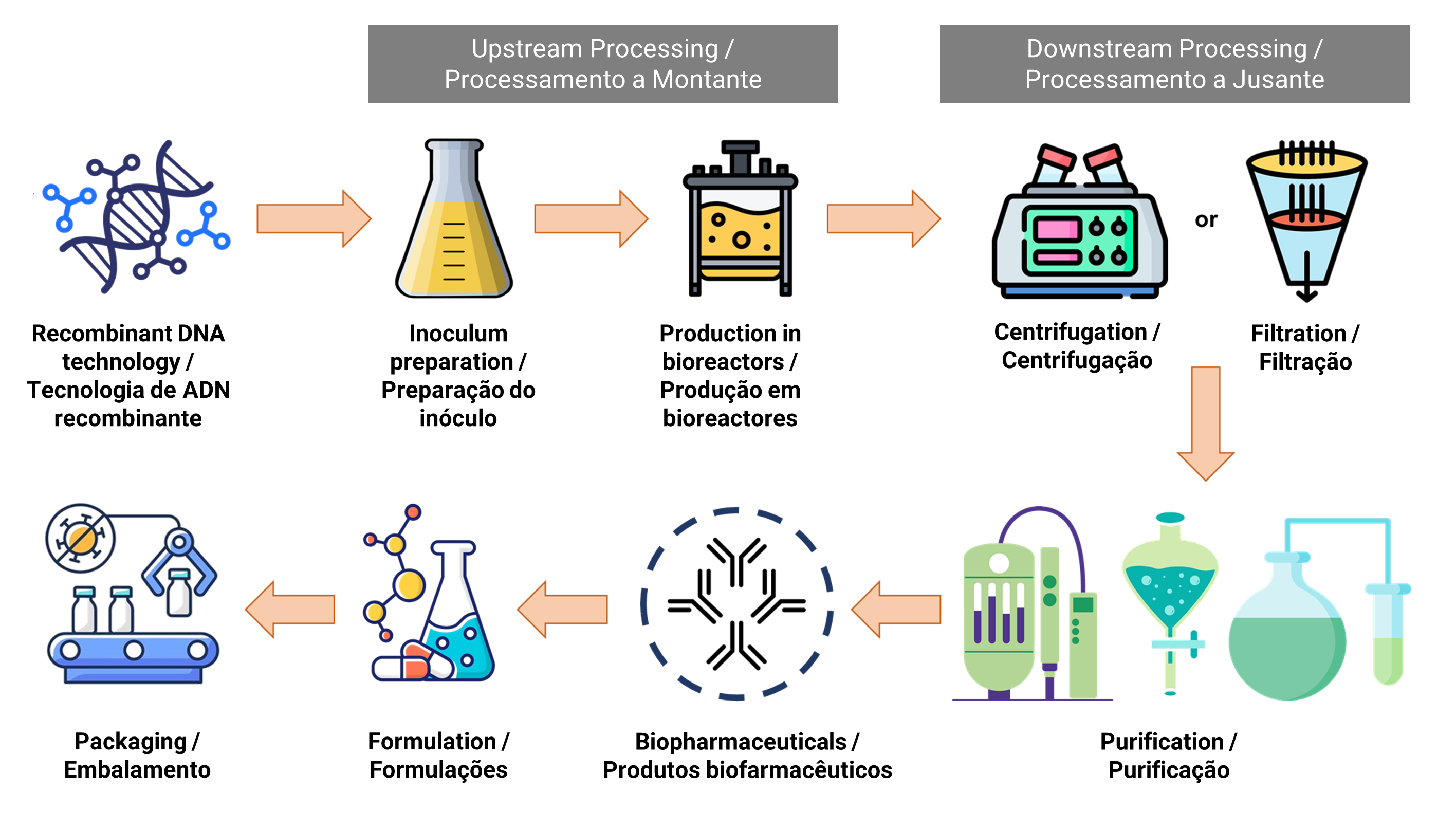 |
Biopharmaceuticals are generally produced through genetically engineered living cells, involving the introduction of DNA sequences into the host cell of the living organism. E. coli stands out as an efficient microorganism for recombinant protein production. By replacing codons rarely found in highly expressed E. coli genes with more favourable major codons, the expression level of the heterologous proteins can be improved (12). Moreover, complex and large therapeutic proteins can be secreted in the periplasm of E. coli, providing an oxidizing environment conducive to forming disulfide bonds that facilitate the proper folding of recombinant proteins (13). Heterologous proteins often accumulate in E. coli as inclusion bodies, which are insoluble misfolded aggregates. For instance, recombinant human insulin has been primarily expressed by E. coli, demonstrating its potential for the treatment of diabetes mellitus type I and II (14).
Additionally, yeast is commonly employed for expressing heterologous proteins that require post-translational modifications for its biological activity, including acetylation, acylation, phosphorylation, O-linked glycosylation and N-linked glycosylation (14). The yeast expression system yields soluble recombinant proteins that are properly folded and functionally active. Furthermore, yeasts can secret proteins to the extracellular medium, thereby facilitating the subsequent purification process (15). Extensive research has been focused on Saccharomyces cerevisiae and Pichia pastoris, both capable of performing human-like N-glycosylation patterns, including the terminal addition of sialic acid to glycoprotein. Yeasts are prominent producers of insulin precursor, human serum albumin, glucagon, hepatitis antigens, vaccine-like particles for various therapeutic applications (16, 17).
Mammalian cell cultures play a significant role in the production of biopharmaceuticals, with Chinese Hamster Ovarian (CHO) cell lines, baby hamster kidney (BHK21) cell lines, and murine myeloma cells being commonly employed (18, 19). Among these, CHO cell lines are particularly popular due to their ability to efficiently express complex therapeutic proteins with human-like glycopatterns, achieving high cell density and sufficient yields. CHO cell lines are capable of growing in chemically defined serum-free medium, making them suitable for high-volume bioreactors and simplifying the downstream processes. Advancements in genetic tools, such as zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs) and the clustered regularly interspaced short palindromic repeat (CRISPR)-associated system have offered opportunities for engineering CHO cell lines, improving protein production and product quality (20). A variety of biopharmaceuticals including human growth hormones, cytokines, mAbs and clotting factors have been successfully produced using CHO cells (19).
Moreover, hybridoma technology provides another option for the production of mAbs, offering convenience, cost-effectiveness, and high production yields (21). Hybridoma cells are generated through the fusion of an activated B lymphocyte with an immortal myeloma cell. Each hybridoma cell consistently expresses a large quantity of one highly specific mAbs, and selected hybridoma clones can be cryopreserved for continuous mAbs production over an extended period. The Hybridoma generation process leverages a host animal's natural ability to produce functional, highly specific, and high affinity mAbs (22). Different animal species including mice, rabbits, guinea pigs, chickens, cows, goats, hamster, and sheep, have been employed for the development of mAbs. The selection of animal species depends on factors like the presence of a homologous protein in the immunized species, the quantity of protein or antigen available for immunization, the availability of suitable fusion partner, the duration needed to achieve an antibody response and the intended purpose for which these mABs are needed (22, 23). Hybridomas are generally categorized into homo-hybridomas and hetero-hybridomas, where IgG-secreting B cells and fusion partners are from the same species and different species, respectively (24).
Following the upstream processes in the production of biopharmaceuticals, downstream processing becomes essential to purify the desired biological products from cell culture broth. This downstream process comprises multiple stages, including primary recovery, purification and polishing, aiming to eliminate both processes- and product-related impurities to ensure the safety of biopharmaceuticals (25). In primary recovery, centrifugation or filtration is employed to separate cells from the supernatant. Subsequently, the sample undergoes concentration, purification, and polishing processes to eliminate the majority of impurities. In cases where biopharmaceuticals are secreted extracellularly in the culture medium, they can undergo the purification process directly. However, for intracellular biopharmaceuticals, cells need to undergo lysis through sonication or a high-pressure homogenizer, followed by clarification to remove cell debris. Additionally, an extra step involving protein refolding through buffer exchange is required to obtain active recombinant proteins if they are expressed as inclusion bodies (26).
Achieving a purity level of greater than 99% in biopharmaceuticals is crucial, and chromatography steps are commonly employed due to their conventional used in protein purification and polishing, offering high resolution capacity (25, 27). Numerous classes of chromatography techniques, such as ion-exchange, affinity, hydrophobic interactions, size exclusion and mix-mode are usually used for biopharmaceuticals purification (28). However, chromatography is often associated with high investment costs and long cycle times, affecting the throughput and scalability of biopharmaceuticals production (29). As alternatives, precipitation (30), microfiltration (31, 32), ultrafiltration (33), crystallization (34), aqueous two-phase system (35, 36), magnetic separation (37, 38) have also been employed for downstream processing of biopharmaceuticals. Currently, downstream processing steps are transitioning from batch to continuous processes, incorporating the use of single-used equipment, improving process control and employing scale-down models for more efficient process development (39, 40).
Biopharmaceutical products and clinical applications
Biopharmaceuticals are now widely used in the treatment of numerous diseases. Several types of biopharmaceutical products including monoclonal antibodies, vaccines, enzymes, hormones, cell therapies, cytokines, growth factors, nucleic acids and carbohydrates are discussed in the following subsections. These diverse categories of biopharmaceuticals play crucial roles in the therapeutic landscape, showcasing the versatility and effectiveness of this class of pharmaceutical products.
Monoclonal antibodies (mAbs)
Monoclonal antibodies (mAbs) are antibodies derived from a single clone of B cells, imparting them with monospecificity and homogeneity, making them well-suited for therapeutic applications (41). Following immunization, each B cell expresses antibodies specific to a particular antigen region (epitope), resulting in slight variations in epitope specificity among the antibodies. The predominant therapeutic mAbs belong to the IgGs due to their extended circulating half-life and ease of production compared to the other classes such as IgM, IgD, IgE and IgA (42). The majority of mAbs therapeutics are employed in the treatment of immunological, oncological and infectious diseases. Table 1 provides a summary of various types of mAbs used for different therapeutic applications, along with their respective targets.
| Table 1. mAbs used for therapeutic applications. |
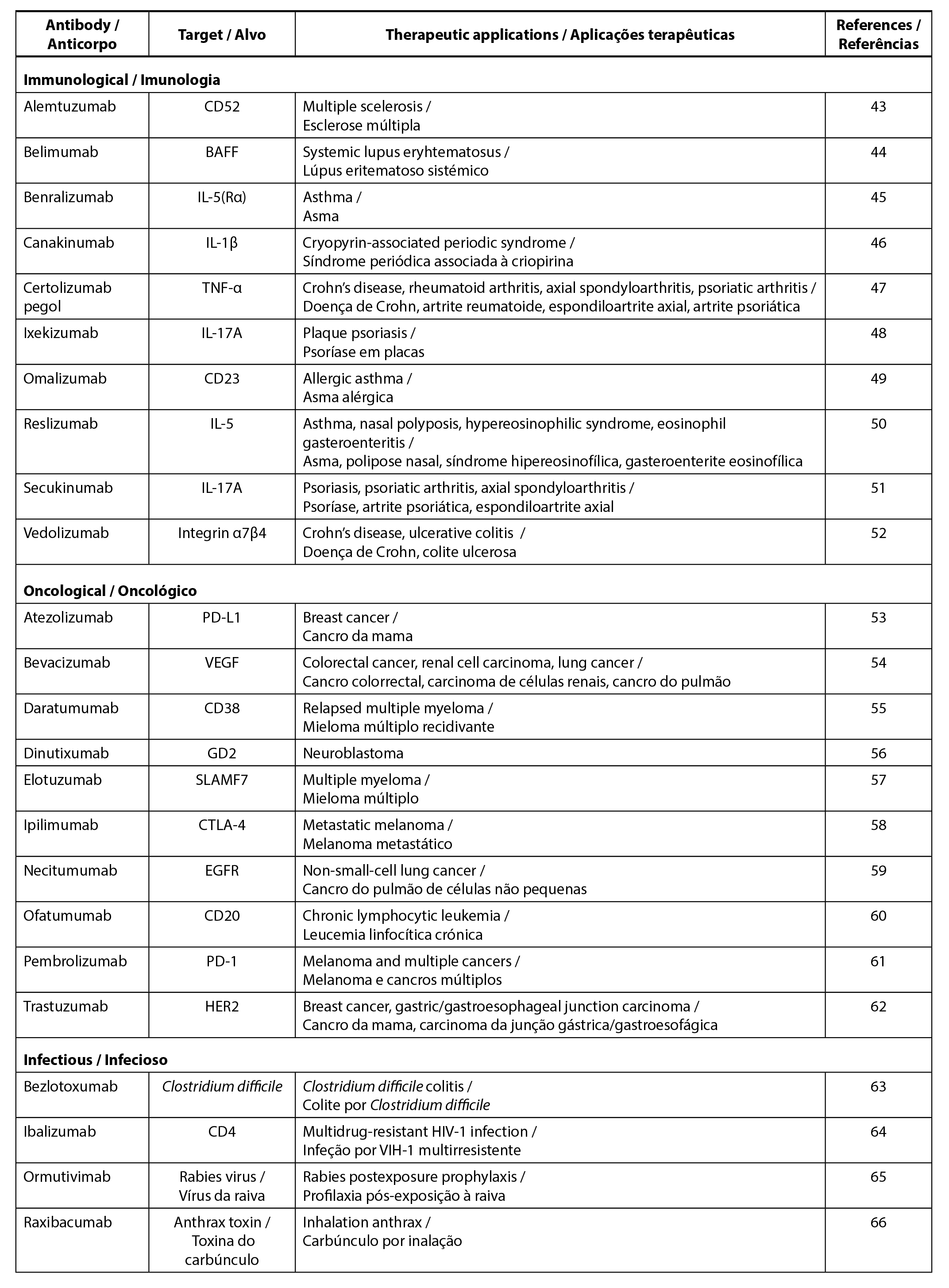 |
In the context of immunological diseases, mAbs target various components of the immune system to minimize excessive responses characteristic of autoimmune diseases. This involves actions such as blocking T cells or B cells, inhibiting the interaction between T cells and antigen-presenting cells, preventing the recruitment of T cells and B cells, hindering T cells differentiation and activation, as well as blocking pro-inflammatory cytokines (67). A notable example of therapeutic mAbs is Adalimumab, the world’s first fully human IgG1 mAbs, which targets TNF-α. Adalimumab is used for the treatment of rheumatoid arthritis, psoriasis, psoriatic arthritis, Crohn’s disease, ulcerative colitis, juvenile idiopathic arthritis and ankylosing spondylitis (10, 68).
Additionally, mAbs-based immunotherapy plays a crucial role in anticancer therapy by targeting tumour antigens and promoting the induction of long-lasting anti-tumour immune responses (69). Therapeutic MAbs mainly target growth factor receptors (e.g. EGFR, HER2, etc.) overexpressed in tumour cells, where blocking these receptors hinders ligand binding or signalling. This, in turn, decreases the tumour growth rate, induces apoptosis and sensitizes tumours to chemotherapy (70). Furthermore, mAbs function to selectively deliver radioisotopes to cancer cells and inhibit angiogenesis by targeting the tumour microenvironment. mAbs-based therapy also targets immune cells by inhibiting immune checkpoints such as cytotoxic T‐lymphocyte associated antigen 4 (CTLA‐4) and programmed cell death protein 1 (PD‐1)/PD1 ligand 1 (PD‐L1), thus enhancing antitumor immune responses (71).
The use of mAbs in the treatment of infectious diseases offers several advantages, including low risk of pathogen transmission, low lot-to-lot variability, and negligible immunological complications associated with the use of heterologous sera (70). Palivizumab, the first approved mAbs for infectious disease, is used for the prevention of respiratory disease resulting from respiratory syncytial virus through the inhibition of virus replication (72). Other mAbs, such as erenumab, fremanezumab and galcanezumab have been employed for the treatment of migraines (73).
Enzymes
Enzymes have emerged as essential biopharmaceuticals, finding applications in enzyme therapy or enzyme replacement therapy for the treatment of disorders that were previously difficult to address (74). These biologically active molecules exhibit significant catalytic potential, characterized by high substrate specificity and affinity, low toxicity, and minimal side effects (75). Enzymatic catalysis enables the conversion of multiple targets into desired products, allowing for the administration of therapeutics in small quantities (76). Microorganisms play a key role in the widespread production of enzymes owing to their physiological, geographic, and genomic diversity. Microbial enzymes have become a promising resource as therapeutic and diagnostic purposes in healthcare sector because of their consistency, economic feasibility, ease of isolation, and potential for product modification and optimization. Moreover, microorganisms can produce enzymes in high yields using cost-effective medium within a short period of time, without the issues related to seasonal fluctuations (77).
Enzymatic anti-inflammatory drugs are employed as alternatives to conventional Non-Steroidal Anti-Inflammatory Drugs (NSAIDS) for the treatment of inflammation (78). Serratiopeptidase, a historically effective anti-inflammatory agent, has been used in the degradation of atherosclerotic plaque, wound healing, reduction of viscosity and thickness of mucus in allergic reactions and surgical pain management. It has demonstrated clinical applications in treating various conditions, including breast disease, Alzheimer’s diseases, sinusitis, hepatitis, lung disorders, atherosclerosis, and uterine fibroids (79). Collagenase is another enzyme that can be used to yield collagen-derived peptides, enhancing macrophage chemotaxis and increasing cytokine secretion, thereby promoting wound healing. Collagenase has found applications in the treatment of Dupuytren’s disease and Peyronie’s disease. Moreover, superoxide dismutase (SODs) represents an anti-inflammatory agent that transform membrane-impermeable superoxide anions into membrane-diffusible hydrogen peroxide, which can then be detoxified by catalase and peroxidase. SODs disrupt the inflammatory cascade by scavenging damaging free radicals, thereby limiting disease progression. These enzymes are incorporated into drug preparations for various conditions, including myocardial ischemia, Peyronie’s disease, colitis, multiple sclerosis, and breast cancer (80).
Enzybiotics constitutes a promising class of unique antibacterials involving enzymes like lysins, autolysins and lysozymes to combat infectious pathogenic bacteria. Lysins, encoded by double-stranded DNA bacteriophages during the lytic cycle, play a crucial role in cleaving covalent bonds in the peptidoglycan layer of bacterial cell walls or destabilizing the bacterial plasma membrane, leading to the death of bacteria (81). Previous studies have employed various lysins such as Cpl-1, Pal, Ply GBS, PlyC, targeting different Streptococcus sp. (75). Autolysins represent another type of lytic enzymes with Lyt A amidase being the first autolysin used as an antibacterial agent for the treatment of Streptococcus pneumoniae (82). Lysozymes, hydrolytic enzymes that specifically cleave β-1,4 glycosidic bonds in the peptidoglycan layer, induce bacterial lysis (83). Lysozymes exhibit anti-inflammatory, anticancer, antibacterial, and antiviral activity, making them suitable for therapeutical applications such as wound healing and killing oral bacteria (84, 85).
Microbial fibrinolytic enzymes demonstrate fibrinolysis, a process that breaks down the fibrin network of blood clots without side effects, thereby restoring normal blood flow in the blood vessels (86). Streptokinase, a type of enzyme-based fibrinolytic drug, exhibits thrombolytic activity by forming an active complex with plasminogen which promotes the cleavage of the Arginine-Valine bond in plasminogen. This results in the formation of proteolytic enzyme plasmin that degrades the matrix of the thrombus, leading to the dissolution of blood clots (75). Another type of fibrinolytic enzyme is known as staphylokinase which possesses similar thrombolytic activity to streptokinase. Nattokinase enzyme exhibits strong fibrinolytic activity and can inactivate Plasminogen activator inhibitor-I (PAI-I) (87). Nattokinase offers advantages over other fibrinolytic enzymes due to its potential for oral administration, sustained effects, stability in gastrointestinal tract and improved plasminogen activators production (88).
Vaccines
Vaccines, a category of biopharmaceuticals, play a crucial role in enhancing the human immune response against various infections or diseases. They can be derived from various components of pathogens, such as surface proteins, nucleic acids, glycoproteins, or biomanufactured (89). Antigens that stimulate the immune system are the main component of vaccines, and additional elements like stabilizers, antibiotics, preservatives, trace components, excipients, and adjuvants are often added to boost immunogenicity and improve shelf life. Prophylactic vaccines are primarily developed for infectious diseases and are commonly administered to healthy individuals to produce antibodies for disease prevention. Prophylactic vaccines have been highly effective against life-threatening diseases such as smallpox and viral poliomyelitis (90). On the other hand, therapeutic vaccines are used as post-exposure therapy to boost the individual’s immune system against chronic disease, premalignant condition or cancer. These vaccines are designed to induce cell-mediated immunity, induce tumour regression, eradicate minimal residual disease (91).
Live attenuated vaccines, a conventional type of vaccine, have demonstrated effectiveness against tuberculosis, chickenpox, influenza, measles, mumps rotavirus and polio (92). In contrast, whole inactivated vaccines, which are safer due to the prevention of replication and mutations, are introduced for diseases like poliovirus, Hepatitis A Virus and Japanese Encephalitis Virus (92). Virus-like particles vaccines are utilized for viruses like Human Papilloma Virus (HPV), Hepatitis B virus, Hepatitis E virus, influenza virus, SARS-CoV-2 virus and respiratory syncytial virus. These vaccines are expressed in different systems including yeast, insect cells and plant (93). Polysaccharide or polysaccharide conjugated vaccines, derived from polymers forming the capsular structure of bacterial pathogens, protect against invasive meningococcal disease, pneumococcal disease, and typhoid (94-96).
Novel vaccines platforms have emerged to enable rapid responses to emerging pathogens, particularly those with pandemic pathogens. These platforms are designed for large-scale manufacturing with low costs, a small footprint and easier deployment. Advances in molecular biology, bioinformatics, and technologies like NexGen sequencing contribute to the development of these platforms. (89). Bacterial-vectored vaccines involve the use of live non-pathogenic bacterial cells, like Lactobacillus sp., as carriers to facilitate mucosal administration of vaccines. Virus-vectored vaccines are derived from viruses engineered to encode genes for antigens cloned into the vector backbone. These vaccines are particularly used for the rapid production of prophylactic vaccines during epidemic or endemic situations (97). An example is JNJ-78436735 by Janssen Biotech Inc., a viral vector vaccine for SARS-CoV-2 virus. DNA vaccines deliver genes encoding immunogenic antigens to the host’s cells using DNA plasmids as a vector, including both humoral and cell-mediated immune response (98). mRNA vaccines, on the other hand, deliver antigen-encoding mRNA into immune cells to trigger an immune response. Examples include COMINARTY® and SpikeVax®, two mRNA vaccines for SARS-CoV-2 virus.
Stem cells
Steam cell therapy involves introducing stem cells into the body cells to induce self-regenerative and differentiation capacity, aiming to regenerate damaged cells and tissues or replace them with new, healthy, and fully functional cells (99). Stem cells are classified as autologous, using the patient’s own cells, or allogeneic, using cells from a healthy donor. Human pluripotent stem cell (hPSCs), characterized by their self-renewable ability, have the potential to differentiate into various cellular phenotypes to treat a wide range of diseases (100). Furthermore, mesenchymal stem cells (MSCs), originating from the early mesoderm and self-renewing mesodermal cells possess multidirectional differentiation potential (101). hPSCs and MSCs are derived from bone marrow, adipose tissue, or umbilical cord for the treatment of human diseases such as pulmonary dysfunctions, metabolic/endocrine-related diseases, neurological disorders, digestive system diseases reproductive disorders, cardiovascular conditions, wound healing, and skin burns.
In addition to hPSCs and MSCs, stem cell-based therapy for liver diseases involves hematopoietic stem cell (HSCs) and liver progenitor cells (102). The administration of MSCs has been proven to reduce bone lesions, enhance bone regeneration, and stimulate the vascularization process in degenerative cartilage, making it a potential treatment for arthritis (102). In cancer treatment, MSCs demonstrate the ability to migrate toward damaged sites through inherent tropism controlled by growth factors, chemokines, and cytokines. The unique capacity of MSCs to regulate tissue repair and recovery contributes to both the protumor and antitumor functions (99). In the generation of burn tissue, MSCs contribute to the generation of keratinocytes and secretion profiles that greatly enhance the skin regeneration process (103).
Human growth hormone
Human growth hormone (hGH), also known as somatotropin, is released into bloodstream and plays a crucial role in numerous biological functions, including the metabolism of carbohydrates, lipids and proteins, lactation, immune regulation, and cell proliferation (104). hGH has diverse effects such as accelerating wound healing, increasing insulin-like growth factor (IGF)-1 osteocalcin, type I pro-collagen pro-peptide (PICP) and bone density, as well as enhancing the absorption of calcium, magnesium, and phosphate in the body (105, 106). Clinically, hGH has been used in the treatment of conditions such as bone fractures, skin burns, AIDS-related wasting, and bleeding ulcers as well as in disorders like Down’s syndrome, Noonan’s syndrome and Prader-Willi syndrome (104, 107). Recombinant hGH exhibits minimal side effects, low cytotoxicity, high selectivity, and low non-specific interactions, making it a valuable therapeutic option (108).
Cytokines
Cytokines, a distinctive class of biopharmaceuticals, are immunoregulatory proteins responsible for regulating cell proliferation, differentiation, and immune responses. Biological therapies for cancer, autoimmune disorders, viral diseases, infectious diseases, inflammatory conditions, and multiple sclerosis often utilize cytokines such as interleukins (IL), interferons (IFNs), and growth factors (109, 110).
Interleukin-2 (IL-2) stands out as a prominent cytokine in tumor therapy such as melanoma and renal cancer, activating, differentiating, and maintaining T cells. Initially, these T cells express a low-affinity dimeric receptor with β- and γ-chains, and upon T cell activation, they acquire the trimeric high-affinity IL-2 receptor (IL-2R), which includes the α-chain (111). Tumor necrosis factor-α (TNF-α), another pro-inflammatory cytokine, acts on endothelial cells to contain infections by increasing vasculature permeability and blood clotting. It also attracts and activates adjacent immune cells, inducing direct apoptosis of certain tumor cells, eliciting hemorrhagic necrosis, and enhancing the influx of immune effector cells. As a result, TNF-α finds extensive use in cancer therapy for the treatment of soft tissue sarcomas and metastatic melanomas (112). IL-12, a different type of cytokine, plays a crucial role in regulating T-cell and natural killer-cell responses. It induces the production of interferon-γ (IFN-γ), promotes the differentiation of T helper 1 (TH1) cells, and serves as a vital link between innate resistance and adaptive immunity (113).
Interferons (IFNs) can be classified into Type I, II, and III based on the type of receptor and amino acid sequence. Among Type I IFNs, IFN-α has received approval for treating multiple myeloma and chronic hepatitis B and C, while IFN-β is employed in the treatment of multiple sclerosis. Type II IFN, IFN-λ, has shown promise as an alternative therapy for conditions such as atherosclerosis, tuberculosis, cancers, fungal infections, and chronic granulomatous disease (114). Type III IFNs induce a more targeted antiviral or immunomodulatory response, displaying antiviral activity against various gastrointestinal and respiratory viruses, including respiratory syncytial virus, influenza virus, rotavirus, metapneumovirus, and coronavirus (115, 116).
Recombinant growth factors exhibit the ability to stimulate cell growth and facilitate the healing of both normal and pathological wounds. Hepatocyte growth factor, for example, has been utilized in the treatment of inflammatory bowel disease, promoting hepatocyte proliferation and modulating intestinal epithelial cell proliferation and migration, leading to the rapid repair of intestinal epithelial cells (117). Additionally, vascular endothelial growth factor and fibroblast growth factor have proven effective in addressing coronary artery disease (118, 119). Insulin-like growth factor signaling in central nervous system-resident macrophages plays a role in regulating the morphology and transcriptome of these cells, thereby mitigating the severity of autoimmune inflammation (120). In the wound healing process, various growth factors act through autocrine, paracrine, or endocrine mechanisms by binding to membrane or cytoplasmic receptors. This initiates a cascade of events that activate cellular machinery to facilitate wound healing. Examples of growth factors used in surgical applications include epidermal growth factor, keratinocyte growth factor, fibroblast growth factor, granulocyte-macrophage colony-stimulating factor, transforming growth factor-beta, platelet-derived growth factor, and vascular endothelial growth factor (121, 122).
Nucleic acids
Nucleic acids (NAs) therapeutics encompass a wide range of DNA and RNA, including oligonucleotides, antisense oligonucleotides, short interfering RNA (siRNA), and mRNA (123). Oligonucleotides refer to short DNA or RNA molecules, while antisense oligonucleotides are short, single-stranded DNA. siRNA consists of small and double-stranded DNA, and mRNA comprises thousands of nucleotides. Table 2 provides examples of NA therapeutics based on biological classification, along with their respective targets and therapeutic applications.
| Table 2. Examples of nucleic acids therapeutics. |
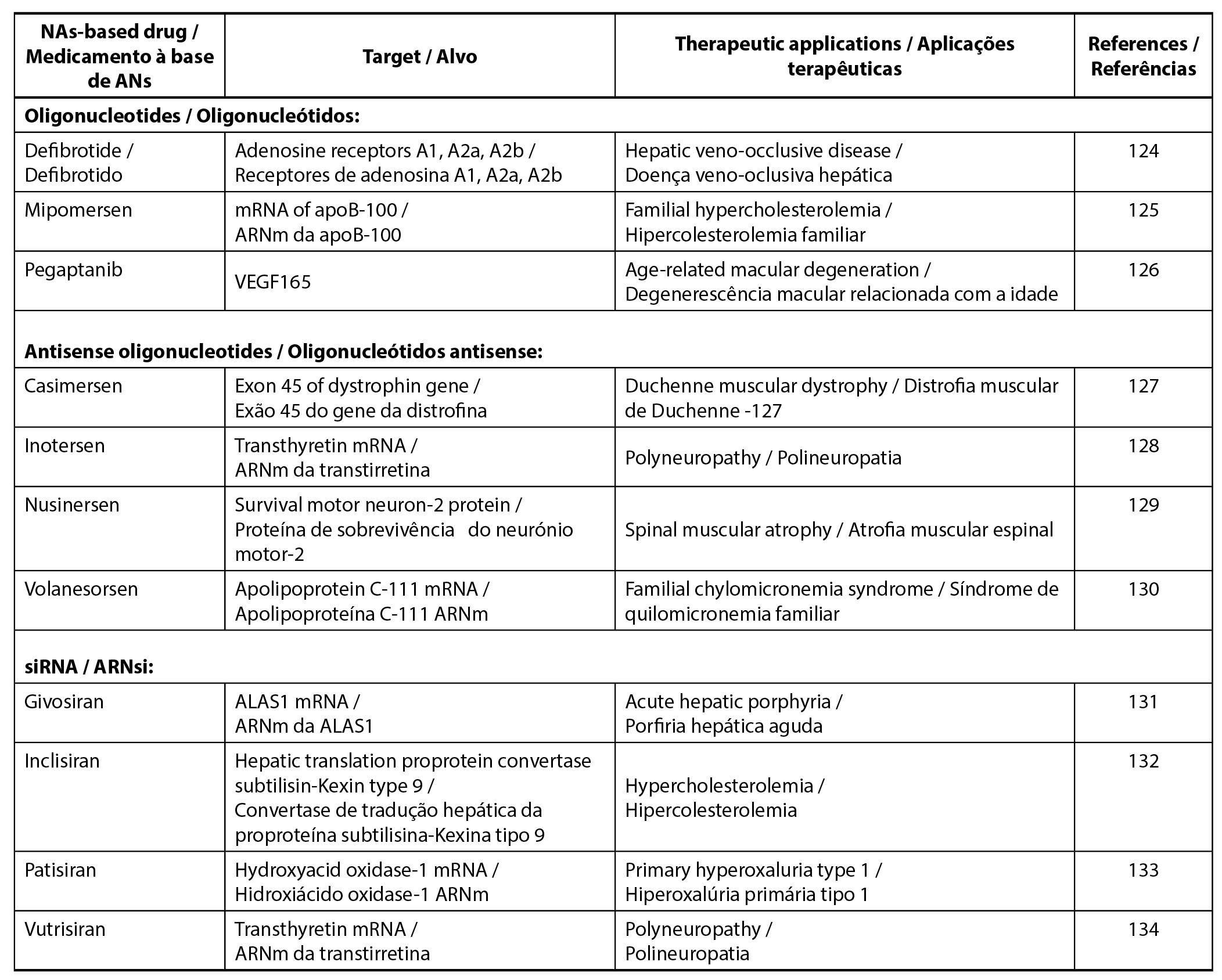 |
NAs are directly administered to target cells or tissues, but their complexity and high hydrophilic often pose stability challenges. Naked and unmodified NAs exhibit short half-lives due to enzymatic and chemical degradation. Therefore, the development of high-quality formulations with effective drug delivery systems is a safeguard to protect NAs from degradation and ensure their successful delivery to target cells or tissues (135). Various options for these therapeutics include lipid-based nanoparticles, polymeric nanoparticles, gold nanoparticles porous nanoparticles. Notably, lipid nanoparticles demonstrate a higher compatibility compared to polymeric and metal-based counterparts (136). For instance, Patisiran (Onpattro®) is the first lipid nanoparticle formulation commercially available, delivered in liposome vesicles (137). Moreover, lipid nanoparticles find application in COVID-19 mRNA vaccines, contributing to enhanced stability and efficiency (135).
Carbohydrates
Carbohydrate-based therapeutics play a significant role in cardiovascular and haematological treatments, addressing conditions including inflammatory diseases, anti-thrombotic treatment, and wound healing. Carbohydrates are characterized by their high density of functional groups, diverse molecular structures, and ideal biocompatibility, as they are ubiquitous in the body (138). Historically, heparin has been utilized as an intravenously injected anticoagulant and antithrombotic agent, crucial in cardiovascular surgeries and haemodialysis (139). Oligosaccharides and polysaccharides find application in the treatment of gastrointestinal tract disease treatment due to their low lipophilicity, where limited absorption is acceptable. Additionally, lactulose is employed in the treatment of chronic constipation by promoting intraluminal gas formation and facilitating bowel movement (140). An example of a pure carbohydrate drug is the 18F-fluorodeoxyglucose (18F-FDG) injection, used for cancer diagnosis as this compound can be taken up by tumor cells, enabling the identification of tumor sites and determining cancer stage (141)
Moreover, carbohydrates can serve as conjugates in other drugs, increasing bioactivity, improving physicochemical properties, and enabling targeted drug delivery. 18F-FDG, for instance, is a valuable component for glycoconjugate targeting in breast, lung, colorectal, and endometrial carcinomas, as well as soft tissues and bone sarcomas (142). Carbohydrates are increasingly used as scaffolds for the development of bioactive compounds, mimicking the backbone of peptides to increase the bioavailability. The first peptidomimetic compound, based on the β-d-glucoside scaffold, exemplifies this approach (143). The application of carbohydrates extends to the development of antimicrobial vaccines and anticancer vaccines, with Prevnar 13 being a typical polysaccharide–protein conjugate vaccine (144). Furthermore, carbohydrates are integrated into nanomaterials for biomedical imaging, diagnostics, and therapeutics to enhance drug efficacy, reduce nonspecific toxicity, and improve targeting (145). Carbohydrates contribute to increased water solubility, improved biocompatibility of nanomaterial and enhanced affinity for receptors, thus optimizing the use of nanomaterials (138).
Challenges
The rapid advancement of innovative biopharmaceuticals has opened a new era, indicating promising scientific and regulatory prospects. However, challenges in biopharmaceutical development persist, including concerns such as the processing cost of biopharmaceuticals, limitations in designing injectable formulations and difficulties in predicting human toxicity based on animal studies.
The manufacturing of biopharmaceuticals is often associated with high cost, primarily due to the utilization of expensive technologies like recombinant DNA technology. Unlike other synthetic and conventional pharmaceutical products, the manufacturing process for biopharmaceuticals is intricate, involving the isolation, growth, and reproduction of living organisms. Downstream processing of biopharmaceutical production accounts for approximately 80% of the overall production costs. This complexity presents challenges in ensuring safety, quality, and efficacy. Additionally, the packaging of biopharmaceuticals must meet stringent requirements to ensure the stability of biological compounds until their administration. These compounds are particularly sensitive to temperature changes and environmental factors. The implementation of continuous processing holds the potential to address these challenges by reducing capital costs, increasing profitability and productivity, and enhancing product quality and flexibility. It is important to conduct economic analyses to assess the cost-effectiveness of continuous processing and explore alternative, cost-effective innovative processes for biopharmaceutical production (146).
Given that injection is the primary administration mode for biopharmaceuticals due to their poor membrane permeation nature, the design of injectable formulation possesses challenges not commonly encountered with other small molecule drugs. The low stability of biopharmaceuticals resulting from structural modification or environmental factors necessitates the use of stabilizers in their formulation. However, careful consideration in terms of local toxicity and potential immunogenicity is essential. Understanding the inactivation mechanism of biopharmaceutical drugs and assessing the suitability of excipients used in the formulation for stabilization are critical. The high and variable viscosity of biopharmaceutical drugs containing multi-hundred-milligram per milliliter protein solutions makes drug administration challenging, thus the creation of low-viscosity formulations is a significant challenge (8).
Animal studies conducted during the development of biopharmaceutical products pose challenges in predicting human toxicity. This difficulty arises from factors such as cross-reactivity, potentially exaggerated pharmacology, and immunogenic responses observed in animals, which may not accurately predict immunogenicity in humans. The prospect of adverse immune reactions may result in clinical consequences, including the risk of anaphylaxis, reduced drug half-life, and the neutralization of both the biopharmaceuticals and their endogenous human analogues. Hence, there is a need for alternatives to animal studies, including in vitro tests such as tissue samples or cell lines, alternative organisms like bacteria, and advanced technologies such as organ-on-chip technologies such as computer modeling or phase 0 in-human microdosing trials (147).
Conclusion
Biopharmaceuticals present numerous advantages in therapeutic applications, effectively contributing to disease prevention, treatment, and diagnosis. The current array of clinical biopharmaceutical products, including mAbs, enzymes, vaccines, stem cells, human growth hormones, cytokines, nucleic acids, and carbohydrates, underscores their versatility and potential impact. This review presents important updated knowledge on biopharmaceuticals, offering insight that can inform decision-making, policy development, and further research in the field of healthcare and pharmaceuticals.
Contribution of the authors
Kai Bin Liew: Conceptualization, Writing - Original draft, Supervision. Siew Keah Lee: Investigation, Writing - Original draft. Long Chiau Ming:Investigation, Writing - Original draft. Zaidul Islam Sarker: Formal analysis, Writing - Original draft. A.B.M. Helal Uddin: Data curation, Writing - Review and editing. Yik Ling Chew: Resources, Writing - Review and editing. Phei Er Kee: Visualization, Writing - Review and editing.
Funding
This work was supported by the Ministry of Higher Education Malaysia (MOHE) Fundamental Research Grant Scheme (FRGS) (grant no: FRGS/1/2021/SKK0/UOC/02/2).
Acknowledgments
We would like to thank all of the bodies and institutions involved that gave us the opportunity and provide their facilities to finish this study.
Conflict of Interests
The authors declare no conflict of interest with regard to the work.
References
1. González Peña, O.I., López Zavala, M. Á., & Cabral Ruelas, H. (2021). Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. International journal of environmental research and public health, 18(5), 2523. 10.3390/ijerph18052532
2. O’Flaherty, R., Bergin, A., Flampouri, E., Mota, L.M., Obaidi, I., Quigley, A., Xie, Y., & Butler, M. (2020). Mammalian Cell Culture for Production of Recombinant Proteins: A review of the Critical Steps in Their Biomanufacturing. Biotechnology advances, 1(43), 107552. 10.1016/j.biotechadv.2020.107552
3. Ghanemi, K., & Yan, S. (2017). Biopharmaceutical Innovation: Benefits and Challenges. Open access journal of science, 1(1), 00004. 10.15406/oajs.2017.01.00004
4. Walsh, G. (2018). Biopharmaceutical Benchmarks 2018. Nature biotechnology, 36(12), 1136-45. 10.1038/nbt.4305
5. Misra, M. (2012). Biosimilars: Current Perspectives and Future Implications. Indian journal of Pharmacology, 44(1), 12-14. 10.4103/0253-7613.91859
6. Kesik-Brodacka, M. (2018). Progress in Biopharmaceutical Development. Biotechnology and applied biochemistry, 65(3), 306-322. 10.1002/bab.1617
7. Skalko-Basnet, N. (2014). Biologics: The Role of Delivery Systems in Improved Therapy. Biologics, 8, 107-114. 10.2147/BTT.S38387
8. Mitragotri, S., Burke, P.A., & Langer, R. (2014). Overcoming the Challenges in Administering Biopharmaceuticals: Formulation and Delivery Strategies. Nature reviews drug discovery, 13(9), 655-672. 10.1038/nrd4363
9. Gronemeyer, P., Ditz, R., & Strube, J. (2014). Trends in Upstream and Downstream Process Development for Antibody Manufacturing. Bioengineering (Basel), 1(4), 188-212. 10.3390/bioengineering1040188
10. Jozala, A.F., Geraldes, D.C., Tundisi, L.L., Feitosa, V.A., Breyer, C.A., Cardoso, S.L., Mazzola, P.G., de Oliveira-Nascimento, L, de Oliveira Rangel-Yagui, de Oliveira Magalhães, P., de Oliveira, M.A., & Jr A.P. (2016). Biopharmaceuticals from Microorganisms: From Production to Purification. Brazalian journal of microbiology, 47 (Suppl 1), 51-63. 10.1016/j.bjm.2016.10.007
11. Tavares, A.P.M., Neves, M.C., Trindade, T., & Freire, M,G. (2020). Recovery and Purification of (Bio)Pharmaceuticals Using (Nano)Materials. Recent advances in analytical techniques, 4, 58-93. 10.2174/9789811405112120040005
12. Zhang, Z.X., Nong, F.T., Wang, Y.Z., Yan, C.X., Gu, Y., Song, P., & Sun, X.-M. (2022). Strategies for Efficient Production of Recombinant Proteins in Escherichia coli: Alleviating the Host Burden and Enhancing Protein Activity. Microbial cell factories, 21(1), 191. 10.1186/s12934-022-01917-y
13. Bhatwa, A., Wang, W., Hassan, Y.I., Abraham, N., Li, X.Z., & Zhou, T. (2021). Challenges Associated With the Formation of Recombinant Protein Inclusion Bodies in Escherichia coli and Strategies to Address Them for Industrial Applications. Frontiers in bioengineering and biotechnology, 9, 630551. 10.3389/fbioe.2021.630551
14. Baeshen, N.A., Baeshen, M.N., Sheikh, A., Bora, R.S., Ahmed, M.M., Ramadan, H.A., Saini, K.S., & Redwan, E.M. (2014). Cell Factories for Insulin Production. Microbial cell factories, 13, 141. 10.1186/s12934-014-0141-0
15. Hou, J., Tyo, K.E.J., Liu, Z., Petranovic, D., & Nielsen, J. (2014). Metabolic Engineering of Recombinant Protein Secretion by Saccharomyces cerevisiae. FEMS yeast research, 12(5), 491-510. 10.1111/j.1567-1364.2012.00810.x
16. Polez, S., Origi, D., Zahariev, S., Guarnaccia, C., Tisminetzky, S.G., Skoko, N., & Baralle, M. (2016). A Simplified and Efficient Process for Insulin Production in Pichia pastoris. PLoS one, 11(12), e0167207. 10.1371/journal.pone.0167207
17. Nielsen, J. (2013). Production of Biopharmaceutical Proteins By Yeast: Advances Through Metabolic Engineering. Bioengineered, 4(4), 207-211. 10.4161/bioe.22856
18. Kantargjieff, A., & Zhou, W. (2013). Mammalian Cell Cultures for Biologics Manufacturing Preface, Advance in biochemical engineering and biotechnology, 139, 1-9. 10.1007/10_2013_255
19. Dumont, J., Euwart, D., Mei, B., Estes, S., & Kshirsagar, R. (2016). Human Cell Lines for Biopharmaceutical Manufacturing: History, Status, and Future Perspectives. Critical reviews in biotechnology. 36(6), 1110-1122. 10.3109/07388551.2015.1084266
20. Glinšek, K., Bozovičar, K., & Bratkovič, T. (2023). CRISPR Technologies in Chinese Hamster Ovary Cell Line Engineering. International journal of molecular sciences, 24(9), 8144. 10.3390/ijms24098144
21. Mitra, S., & Tomar, P.C. (2021). Hybridoma Technology; Advancements, Clinical Significance, and Future Aspects. Journal of genetic engineering and biotechnology, 19(1), 159. 10.1186/s43141-021-00264-6
22. Parray, H.A., Shukla, S., Samal, S., Shrivastava, T., Ahmed, S., Sharma, C., & Kumar, R. (2020). Hybridoma Technology A Versatile Method for Isolation of Monoclonal Antibodies, Its Applicability Across Species, Limitations, Advancement and Future Perspectives. International immunopharmacology, 85, 106639. 10.1016/j.intimp.2020.106639
23. Muyldermans, S., & Smider, V.V. (2016). Distinct Antibody Species: Structural Differences Creating Therapeutic Opportunities. Current opinion in immunology, 40, 7-13. 10.1016/j.coi.2016.02.003
24. Weber, J., Peng, H., & Rader, C. (2017). From Rabbit Antibody Repertoires To Rabbit Monoclonal Antibodies. Experimental & molecular medicine, 49(3), e305. 10.1038/emm.2017.23
25. Fields, C., Li, P., O'Mahony, J.J. & Lee, G.U. (2106). Advances in Affinity Ligand-Functionalized Nanomaterials for Biomagnetic Separation. Biotechnology & bioengineering, 113(1), 11-25. 10.1002/bit.25665
26. Yamaguchi, H., & Miyazaki, M. (2014). Refolding Techniques for Recovering Biologically Active Recombinant Proteins from Inclusion Bodies. Biomolecules, 4(1), 235-251. 10.3390/biom4010235
27. Liu, H.F., Ma, J., Winter, C., & Bayer, R. (2010). Recovery and Purification Process Development for Monoclonal Antibody Production. MAbs, 2(5), 480-499. 10.4161/mabs.2.5.12645
28. Faria, R.P.V., & Rodrigues, A.E. (2015). Instrumental Aspects of Simulated Moving Bed chromatography. Journal of chromatography A, 1421, 82-102. 10.1016/j.chroma.2015.08.045
29. Rosa, P.A.J., Ferreira, I.F., Azevedo, A.M., & Aires-Barros, M.R. (2010). Aqueous Two-Phase Systems: A Viable Platform in the Manufacturing of Biopharmaceuticals. Journal of chromatography A, 1217(16), 2296-2305. 10.1016/j.chroma.2009.11.034
30. Dutra, G., Komuczki, D., Jungbauer, A., & Satzer, P. (2020). Continuous Capture of Recombinant Antibodies by ZnCl2 Precipitation Without Polyethylene Glycol. Engineering in life sciences, 20(7), 265-274. 10.1002/elsc.201900160
31. Burgstaller, D., Jungbauer, A., & Satzer, P. (2019). Continuous Integrated Antibody Precipitation With Two-Stage Tangential Flow Microfiltration Enables Constant Mass Flow. Biotechnology and bioengineering, 116(5), 1053-1065. 10.1002/bit.26922
32. Li, Z., Gu, Q., Coffman, J.L., Przybycien, T., & Zydney, A.L. (2019). Continuous Precipitation For Monoclonal Antibody Capture Using Countercurrent Washing by Microfiltration. Biotechnology progress, 235(6), e2886. 10.1002/btpr.2886
33. Thakur, G., & Rathore, A.S. (2021). Modelling and Optimization of Single-Pass Tangential Flow Ultrafiltration for Continuous Manufacturing of Monoclonal Antibodies. Separation and purification technology, 276, 119341. 10.1016/j.seppur.2021.119341
34. Chen, W., Li, X., Guo, M., Link, F.J., Ramli, S.S., Ouyang, J., Rosbottom, I., & Heng, J.Y.Y. (2021). Biopurification of Monoclonal Antibody (mAb) Through Crystallisation. Separation and purification technology, 263, 118358. 10.1016/j.seppur.2021.118358
35. Kruse, T., Kampmann, M., Rüddel, I., & Greller, G. (2020). An Alternative Downstream Process Based on Aqueous Two-Phase Extraction for the Purification of Monoclonal Antibodies. Biochemical engineering journal, 161, 107703. 10.1016/j.bej.2020.107703
36. Ornelas-González, A., Reisenauer, S.U., González-González, M., & Rito-Palomares, M. (2020). Characterization and Optimization of Immunoaffinity Aqueous Two-Phase Systems with PEGylated CD133/2-Biotin Antibody in Route to Stem Cell Separation. Journal of chemical technology & biotechnology, 95(1), 123-131. https://doi.org/10.1002/jctb.6213
37. Zanker, A.A., Stargardt, P., Kurzbach, S.C., Turrina, C., Mairhofer, J., Schwaminger, S.P., & Borensmeier, C. (2022). Direct Capture and Selective Elution of A Secreted Polyglutamate-tagged Nanobody Using Bare Magnetic Nanoparticles. Biotechnology journal, 17(5):2100577. 10.1002/biot.202100577
38. Schwaminger, S.P., Blank-Shim, S.A., Scheifele, I., Pipich, V., Fraga-García, P., & Berensmeier, S. (2019). Design of Interactions Between Nanomaterials and Proteins: A Highly Affine Peptide Tag to Bare Iron Oxide Nanoparticles for Magnetic Protein Separation. Biotechnology journal, 14(3), 1800055. 10.1002/biot.201800055
39. Gerstweiler, L., Bi, J., Middelberg, A.P.J. (2021). Continuous Downstream Bioprocessing for Intensified Manufacture of Biopharmaceuticals and Antibodies. Chemical engineering science, 231, 116272. 10.1016/j.ces.2020.116272
40. Strube, J., Ditz, R., Kornecki, M., Huter, M., Schmidt, A., Thiess, H., & Zobel-Roos, S. (2018). Process Intensification in Biologics Manufacturing. Chemical engineering and processing - process intensification, 133, 278-293. 10.1016/j.cep.2018.09.022
41. Lu, R.-M., Hwang, Y.-C., Liu, I.J., Lee, C.-C., Tsai, H.-Z., Li, H.-J., & Wu, H.-C. (2020). Development of Therapeutic Antibodies for the Treatment of Diseases. Journal of biomedical science, 27(1), 1. 10.1186/s12929-019-0592-z
42. Ryman, J.T., & Meibohm, B. (2017). Pharmacokinetics of Monoclonal Antibodies. CPT Pharmacometrics & Systems Pharmacology, 6(9), 576-588. 10.1002/psp4.12224
43. Hu, Y., Turner, M.J., Shields, J., Gale, M.S., Hutto, E., Roberts, B.L., Siders, W.M., & Kaplan, J.M. (2009). Investigation of the Mechanism of Action of Alemtuzumab in A Human CD52 Transgenic Mouse Model. Immunology, 128(2), 260-270. 10.1111/j.1365-2567.2009.03115.x
44. Ramsköld, D., Parodis, I., Lakshmikanth, T., Sippl, N., Khademi, M., Chen, Y., Zickert, A., Mikeš, J., Achour, A., Amara, K., Piehl, F., Brodin, P., Gunnarsson, I., & Malmström, V. (2019). B Cell Alterations During BAFF Inhibition With Belimumab in SLE. EBioMedicine, 40, 517-527. 10.1016/j.ebiom.2018.12.035
45. Ghazi, A., Trikha, A., Calhoun, W.J. (2012). Benralizumab--A Humanized mAb to IL-5Rα With Enhanced Antibody-Dependent Cell-Mediated Cytotoxicity--A Novel Approach for the Treatment of Asthma. Expert opinion biological therapy, 12(1), 113-118. 10.1517/14712598.2012.642359
46. Kuemmerle-Deschner, J.B., & Haug, I. (2013). Canakinumab in Patients With Cryopyrin-Associated Periodic Syndrome: An Update for Clinicians. Therapeutic advances in musculoskeletal disease, 5(6), 315-329. 10.1177/1759720X13502629
47. Curtis, J.R., Mariette, X., Gaujoux-Viala, C., Blauvelt, A., Kvien, T.K., Sandborn, W.J., Winthrop, K., de Longueville, M., Huybrechts, I., & Bykerk, V.P. (2019). Long-Term Safety of Certolizumab Pegol in Rheumatoid Arthritis, Axial Spondyloarthritis, Psoriatic Arthritis, Psoriasis and Crohn's Disease: A Pooled Analysis of 11 317 Patients Across Clinical Trials. RMD open, 5(1), e000942. 10.1136/rmdopen-2019-000942
48. Demirel Öğüt, N., Koç Yıldırım, S., Erbağcı, E., & Hapa, F.A. (2022). Ixekizumab Treatment in Patients With Moderate-To-Severe Plaque Psoriasis in A Real-World Clinical Setting. Journal of cosmetic dermatology, 21(11), 6215-6224. 10.1111/jocd.15217
49. Gon, Y., Maruoka, S., & Mizumura, K. (2022). Omalizumab and IgE in the Control of Severe Allergic Asthma. Frontiers in pharmacology, 13, 839011. 10.3389/fphar.2022.839011
50. Walsh, G.M. (2013). Profile of Reslizumab in Eosinophilic Disease and Its Potential in the Treatment of Poorly Controlled Eosinophilic Asthma. Biologics, 7, 7-11. 10.2147/BTT.S30133
51. Kolbinger, F., Di Padova, F., Deodhar, A., Hawkes, J.E., Huppertz, C., Kuiper, T., Mclnnes, I.B., Ritchlin, C.T., Rosmarin, D., Schett, G., Carballido, J.M., Häusermann, P., Calonder, C., Vogel, B., Rondeau, J.-M., & Bruin, G. (2022). Secukinumab for the Treatment of Psoriasis, Psoriatic Arthritis, and Axial Spondyloarthritis: Physical and Pharmacological Properties Underlie the Observed Clinical Efficacy and Safety. Pharmacology & therapeutics, 229, 107925. 10.1016/j.pharmthera.2021.107925
52. Cherry, L.N., Yunker, N.S., Lambert, E.R., Vaughan, D. & Lowe, D.K. (2015). Vedolizumab: An α4β7 Integrin Antagonist for Ulcerative Colitis and Crohn's Disease. Therapeutic advances in chronic disease, 6(5), 224-233. 10.1177/2040622315586970
53. Ameri, A., Tavakoli-Far, B., Rostami, M., Abedi Kiasari, B., Sakhaei, D., Saad Ahmed, O., Forouzani, F., & Fazli, Y. (2022). Recent Advances in Atezolizumab-Based Programmed Death-Ligand 1 (PD-L1) Blockade Therapy for Breast Cancer. International immunopharmacology, 113(Pt A), 109334. 10.1016/j.intimp.2022.109334
54. Hegde, P.S., Jubb, A.M., Chen, D., Li, N.F., Meng, Y.G., Bernaards, C., Elliott, R., Scherer, S.J., & Chen, D.S. (2013). Predictive Impact of Circulating Vascular Endothelial Growth Factor in Four Phase III Trials Evaluating Bevacizumab. Clinical cancer research, 19(4), 929-937. 10.1158/1078-0432.CCR-12-2535
55. Raedler, L.A. (2016). Darzalex (Daratumumab): First Anti-CD38 Monoclonal Antibody Approved for Patients with Relapsed Multiple Myeloma. American health & drug benefits, 9(Spec Feature), 70-73. 27668047
56. Ploessl, C., Pan, A., Maples, K.T., & Lowe, D.K. (2016). Dinutuximab: An Anti-GD2 Monoclonal Antibody for High-Risk Neuroblastoma. Annals of pharmacotherapy, 50(5), 416-422. 10.1177/1060028016632013
57. Wang, Y., Sanchez, L., Siegel, D.S., & Wang, M.L. (2016). Elotuzumab for the Treatment of Multiple Myeloma. Journal of hematology & oncology, 9(1), 55. 10.1186/s13045-016-0284-z
58. Savoia, P., Astrua, C., & Fava, P. (2016). Ipilimumab (Anti-Ctla-4 Mab) in the Treatment of Metastatic Melanoma: Effectiveness and Toxicity Management. Human Vaccines & Immunotherapeutics, 12(5), 1092-1101. 10.1080/21645515.2015.1129478
59. Thakur, M.K. & Wozniak, A.J. (2017). Spotlight on Necitumumab in the Treatment of Non-Small-Cell Lung Carcinoma. Lung Cancer (Auckl), 8, 13-19. 10.2147/LCTT.S104207
60. Barth, M.J., & Czuczman, M.S. (2013). Ofatumumab: A Novel, Fully Human Anti-CD20 Monoclonal Antibody for the Treatment of Chronic Lymphocytic Leukemia. Future oncology, 9(12), 1829-1839. 10.2217/fon.13.219
61. Burns, M.C., O'Donnell, A., & Puzanov, I. (2016). Pembrolizumab for the Treatment of Advanced Melanoma. Expert opinion on orphan drugs, 4(8), 867-873. 10.1080/21678707.2016.1191348
62. Ross, J.S., & Mulcahy, M. (2011). HER2 Testing in Gastric/Gastroesophageal Junction Adenocarcinomas: Unique Features of a Familiar Test. Gastrointestinal Cancer Research, 4(2), 62-66. 10.1152/ajpcell.00650.2009
63. Navalkele, B.D., & Chopra, T. (2018). Bezlotoxumab: An Emerging Monoclonal Antibody Therapy for Prevention of Recurrent Clostridium difficile Infection. Biologics, 12, 11-21. 10.2147/BTT.S127099
64. Emu, B., Fessel, J., Schrader, S., Kumar, P., Richmond, G., Win, S., Weinheimer, S., Marsolais, C., & Lewis, S. (2018). Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. The new England journal of medicine, 379(7), 645-654. 10.1056/NEJMoa1711460
65. Liu, X., Li, Y., Li, J., Zhou, J., Guo, J., Pu, Y., Jiang, Y., Zhou, Y., Jiang, Z., Shu, Q., Wang, C., Wang, J., Zhao, Y., Zhao, W., Wang, H., Wei, J., Yu, H., Gao, J., & Li, X. (2023). Comparing Recombinant Human Rabies Monoclonal Antibody (Ormutivimab) With Human Rabies Immunoglobulin (HRIG) for Postexposure Prophylaxis: A Phase III, Randomized, Double-blind, Non-inferiority Trial. International journal of infectious diseases, 134, 53-62. 10.1016/j.ijid.2023.05.017
66. Kummerfeldt, C.E. (2014). Raxibacumab: Potential Role in the Treatment of Inhalational Anthrax. Infection and drug resistance, 7, 101-109. 10.2147/IDR.S47305
67. Bruno, V., Battaglia, G., & Nicoletti, F. (2011). The Advent of Monoclonal Antibodies in the Treatment of Chronic Autoimmune Diseases. Neurological Sciences, 31 Suppl 3, 283-288. 10.1007/s10072-010-0382-6
68. Ellis, C.R., & Azmat, C.E. (2023). Adalimumab. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC.
69. Zahavi, D., & Weiner, L. (2020). Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel), 9(3), 34. 10.3390/antib9030034
70. Castelli, M.S., McGonigle, P., & Hornby, P.J. (2019). The Pharmacology and Therapeutic Applications of Monoclonal Antibodies. Pharmacology research & perspect, 7(6), e00535. 10.1002/prp2.535
71. Lee, J.Y., Lee, H.T., Shin, W., Chae, J., Choi, J., Kim, S.H., Lim, H., Heo, T.W., Park, K.Y., Lee, Y.J., Ryu, S.E., Son, J.Y., Lee, J.U., & Heo, Y.-S. (2016). Structural Basis of Checkpoint Blockade by Monoclonal Antibodies in Cancer Immunotherapy. Nature communications, 7(1), 13354. 10.1038/ncomms13354
72. Rogovik, A.L., Carleton, B., Solimano, A., & Goldman, R.D. (2010). Palivizumab for the prevention of respiratory syncytial virus infection. Canadian family physician, 56(8), 769-772.
73. Aditya, S., & Rattan, A. (2023). Advances in CGRP Monoclonal Antibodies as Migraine Therapy: A Narrative Review. Saudi journal of medicine & medical sciences, 11(1), 11-18. 10.4103/sjmms.sjmms_95_22
74. Li, M. (2018). Enzyme Replacement Therapy: A Review and Its Role in Treating Lysosomal Storage Diseases. Pediatric Annals, 47(5), e191-e197. 10.3928/19382359-20180424-01
75. Vachher, M., Sen, A., Kapila, R., & Nigam, A. (2021). Microbial Therapeutic Enzymes: A Promising Area of Biopharmaceuticals. Current research in biotechnology, 3, 195-208. 10.1016/j.crbiot.2021.05.006
76. Yari, M., Ghoshoon, M.B., Vakili, B., & Ghasemi, Y. (2017). Therapeutic Enzymes: Applications and Approaches to Pharmacological Improvement. Current Pharmaceutical Biotechnology, 18(7), 531-540. 10.2174/1389201018666170808150742
77. Gurung, N., Ray, S., Bose, S., & Rai, V. (2013). A Broader View: Microbial Enzymes and Their Relevance in Industries, Medicine, and Beyond. Biomed research international, 2013, 329121. 10.1155/2013/329121
78. Tiwari, M. (2017). The Role of Serratiopeptidase in the Resolution of Inflammation. Asian journal of pharmaceutical sciences, 12(3), 209-215. 10.1016/j.ajps.2017.01.003
79. Jadhav, S.B., Shah, N., Rathi, A., Rathi, V., & Rathi, A. (2020). Serratiopeptidase: Insights into the Therapeutic Applications. Biotechnol reports, 28, e00544. 10.1016/j.btre.2020.e00544
80. Ünlü, A.E., & Takaç, S. Improvement of Superoxide Dismutase Activity Using Experimental Design and Radical Promoters. Biotechnology & biotechnological equipment, 31(5), 1046-1054. 10.1080/13102818.2017.1353923
81. Ghose, C., & Euler, C.W. (2020). Gram-Negative Bacterial Lysins. Antibiotics (Basel), 9(2), 74. 10.3390/antibiotics9020074
82. Rodríguez-Cerrato, V., García, P., Huelves, L., García, E., Del Prado, G., Gracia, M., Ponte, C., López, R., & Soriano, F. (2007). Pneumococcal LytA Autolysin, A Potent Therapeutic Agent in Experimental Peritonitis-Sepsis Caused by Highly Beta-Lactam-Resistant Streptococcus pneumoniae. Antimicrobial Agents and Chemotherapy, 51(9), 3371-3373. 10.1128/AAC.00137-07
83. Nawaz, N., Wen, S., Wang, F., Nawaz, S., Raza, J., Iftikhar, M., & Usman, M. (2022). Lysozyme and Its Application as Antibacterial Agent in Food Industry. Molecules, 27(19), 6305. 10.3390/molecules27196305
84. Gil-Montoya, J.A., Guardia-López, I., & González-Moles, M.A. (2008). Evaluation of the Clinical Efficacy of A Mouthwash And Oral Gel Containing the Antimicrobial Proteins Lactoperoxidase, Lysozyme and Lactoferrin in Elderly Patients with Dry Mouth – A Pilot Study. Gerodontology, 25(1), 3-9. 10.1111/j.1741-2358.2007.00197.x
85. Chen, Q., Li, W., Wang, J., Qu, X., & Wang, G. (2018). Lysozyme-Antimicrobial Peptide Fusion Protein Promotes the Diabetic Wound Size Reduction in Streptozotocin (STZ)-Induced Diabetic Rats. Medical science monitor, 24, 8449-8458. 10.12659/MSM.912596
86. Altaf, F., Wu, S., & Kasim, V. (2021). Role of Fibrinolytic Enzymes in Anti-Thrombosis Therapy. Frontiers in Molecular Biosciences, 8, 680397. 10.3389/fmolb.2021.680397
87. Chen, H., McGowan, E.M., Ren, N., Lal, S., Nassif, N., Shad-Kaneez, F., Qu, X., & Lin, Y. (2018). Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark Insights, 13, 1177271918785130. 10.1177/1177271918785130
88. Kurosawa, Y., Nirengi, S., Homma, T., Esaki, K., Ohta, M., Clark, J.F., Hamaoka, T. (2015). A Single-Dose of Oral Nattokinase Potentiates Thrombolysis and Anti-Coagulation Profiles. Scientific reports, 5(1), 11601. 10.1038/srep11601
89. Ghattas, M., Dwivedi, G., Lavertu, M., & Alameh, M.G. (2021). Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines (Basel), 9(12), 1490. 10.3390/vaccines9121490
90. Kayser, V., & Ramzan, I. (2021). Vaccines and Vaccination: History and Emerging Issues. Human vaccines & immunotherapeutics, 17(12), 5255-5268. 10.1080/21645515.2021.1977057
91. Saxena, M., van der Burg, S.H., Melief, C.J.M., & Bhardwaj, N. (2021). Therapeutic Cancer Vaccines. Nature reviews cancer, 21(6), 360-378. 10.1038/s41568-021-00346-0
92. Aldossary, A.M., Ekweremadu, C.S.M., Offe, I.M., Alfassam, H.A., Han, S., Onyali, V.C., Ozoude, C.H., Ayeni, E.A., Nwagwu, C.S., Halwani, A.A., Almozain, N.H., & Tawfil, E.A. (2022). A Guide to Oral Vaccination: Highlighting Electrospraying as A Promising Manufacturing Technique Toward a Successful Oral Vaccine Development. Saudi pharmaceutical journal, 30(6), 655-668. 10.1016/j.jsps.2022.03.010
93. Nooraei, S., Bahrulolum, H., Hoseini, Z.S., Katalani, C., Hajizade, A., Easton, A.J., & Ahmadian, G. (2021). Virus-Like Particles: Preparation, Immunogenicity and Their Roles as Nanovaccines and Drug Nanocarriers. Journal of nanobiotechnology, 19(1), 59. 10.1186/s12951-021-00806-7
94. Milligan, R., Paul, M., Richardson, M., & Neuberger, A. (2018). Vaccines for Preventing Typhoid Fever. Cochrane database of systematic reviews, 5(5), Cd001261. 10.1002/14651858.CD001261.pub4
95. Clark, S.A., & Borrow, R. (2020). Herd Protection against Meningococcal Disease through Vaccination. Microorganisms, 8(11), 1675. 10.3390/microorganisms8111675
96. Daniels, C.C., Rogers, P.D., & Shelton, C.M. (2016). A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. The journal of pediatric pharmacology and therapeutics, 21(1), 27-35. 10.5863/1551-6776-21.1.27
97. Wang, S., Liang, B., Wang, W., Li, L., Feng, N., Zhao, Y., Wang, T., Yan, F., Yang, S., & Xia, Z. (2023). Viral Vectored Vaccines: Design, Development, Preventive and Therapeutic Applications in Human Diseases. Signal transduction and targeted therapy, 8(1), 149. 10.1038/s41392-023-01408-5
98. Silveira, M.M., Moreira, G.M.S.G., & Mendonça, M. (2021). DNA Vaccines Against COVID-19: Perspectives and Challenges. Life sciences, 267, 118919. 10.1016/j.lfs.2020.118919
99. Hoang, D.M., Pham, P.T., Bach, T.Q., Ngo, A.T.L., Nguyen, Q.T., Phan, T.T.K., Nguyen, G.H., Le, P.T.T., Hoang, V.T., Forsyth, N.R., Heke, M., & Nguyen, L.T. (2022). Stem Cell-Based Therapy for Human Diseases. Signal transduction and targeted therapy, 7(1), 272. 10.1038/s41392-022-01134-4
100. Cabral, J.M.S., da Silva, C.L., & Diogo, M.M. (2020). Stem Cell Bioprocessing and Manufacturing. Bioengineering (Basel), 7(3), 84. 10.3390/bioengineering7030084
101. Jin, Y., Li, S., Yu, Q., Chen, T., & Liu, D. (2020). Application of Stem Cells in Regeneration Medicine. MedComm, 4(4), e291. 10.1002/mco2.291
102. Wang, J., Sun, M., Liu, W., Li, Y., & Li, M. (2019). Stem Cell-Based Therapies for Liver Diseases: An Overview and Update. Tissue engineering and regenerative medicine, 16(2), 107-118. 10.1007/s13770-019-00178-y
103. Li, L., Ngo, H.T.T., Hwang, E., Wei, X., Liu, Y., Liu, J., & Yi, T.-H. (2019). Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. International journal of molecular sciences, 21(1), 49. 10.3390/ijms21010049
104. Rezaei, M., & Zarkesh-Esfahani, S.H. (2012). Optimization of Production of Recombinant Human Growth Hormone in Escherichia coli. Journal of research in medical sciences, 17(7), 681-685. PMC3685787
105. Gilpin, D.A., Barrow, R.E., Rutan, R.L., Broemeling, L., & Herndon, D.N. (1994). Recombinant Human Growth Hormone Accelerates Wound Healing in Children With Large Cutaneous Burns. Annals of surgery, 220(1), 19-24. 10.1097/00000658-199407000-00004
106. Rigi, G., Rostami, A., Ghomi, H., Ahmadian, G., Mirbagheri, V.S., Jeiranikhameneh, M., Vahed, M., & Rahimi, S. (2021). Optimization of Expression, Purification and Secretion of Functional Recombinant Human Growth Hormone in Escherichia coli Using Modified Staphylococcal Protein A Signal Peptide. BMC biotechnology, 21(1), 51. 10.1186/s12896-021-00701-x
107. Annerén, G., Tuvemo, T., Carlsson-Skwirut, C., Lönnerholm, T., Bang, P., Sara, V.R., & Gustafsson, J. (1999). Growth Hormone Treatment in Young Children With Down's Syndrome: Effects on Growth and Psychomotor Development. Archives of disease in childhood, 80(4), 334-338. 10.1136/adc.80.4.334
108. Frokjaer, S., & Otzen, D.E. (2015). Protein Drug Stability: A Formulation Challenge. Nature reviews drug discovery, 4(4), 298-306. 10.1038/nrd1695
109. Lipiäinen, T., Peltoniemi, M., Sarkhel, S., Yrjönen, T., Vuorela, H., Urtti, A., & Juppo, A. (2015). Formulation and Stability of Cytokine Therapeutics. Journal of pharmaceutical sciences, 104(2), 307-326. 10.1002/jps.24243
110. Murer, P., & Neri, D. (2019). Antibody-Cytokine Fusion Proteins: A Novel Class of Biopharmaceuticals for the Therapy of Cancer and of Chronic Inflammation. New biotechnology, 52, 42-53. 10.1016/j.nbt.2019.04.002
111. Arenas-Ramirez, N., Woytschak, J., & Boyman, O. (2015). Interleukin-2: Biology, Design and Application. Trends in immunology, 36(12), 763-777. 10.1016/j.it.2015.10.003
112. Wang, X., & Lin, Y. (2008). Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta pharmacologica sinica, 29(11), 1275-1288. 10.1111/j.1745-7254.2008.00889.x
113. Trinchieri, G. (2003). Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nature reviews immunology, 3(2), 133-146. 10.1038/nri1001
114. Kak, G., Raza, M., & Tiwari, B.K. (2018). Interferon-Gamma (IFN-γ): Exploring Its Implications in Infectious Diseases. Biomolecular concepts, 9(1), 64-79. 10.1515/bmc-2018-0007
115. Shaldzhyan, A., Zabrodskaya, Y., Yolshin, N., Kudling, T., Lozhkov, A., Plotnikova, M., Ramsay, E., Taraskin, A., Nekrasov, P, Grudinin, M., & Vasin, A. (2021). Clean and Folded: Production of Active, High Quality Recombinant Human Interferon-λ1. Process biochemistry, 111, 32-39. 10.1016/j.procbio.2021.08.029
116. Hermant, P., & Michiels, T. (2014). Interferon-λ in the Context of Viral Infections: Production, Response and Therapeutic Implications. Journal of innate immunity, 6(5), 563-574. 10.1159/000360084
117. Ido, A., Numata, M., Kodama, M., Tsubouchi, H. (2005). Mucosal Repair and Growth Factors: Recombinant Human Hepatocyte Growth Factor as An Innovative Therapy for Inflammatory Bowel Disease. Journal of gastroenterology, 40(10), 925-931. 10.1007/s00535-005-1705-x
118. Henry, T.D., Rocha-Singh, K., Isner, J.M., Kereiakes, D.J., Giordano, F.J., Simons, M, Losordo, D.W., Hendel, R.C., Bonow, R.O., Eppler, S.M., Zioncheck, T.F., Holmgren, E.B., & McCluskey, E.R. (2001). Intracoronary Administration of Recombinant Human Vascular Endothelial Growth Factor to Patients With Coronary Artery Disease. American heart journal, 142(5), 872-880. 10.1067/mhj.2001.118471
119. Simons, M., Annex, B.H., Laham, R.J., Kleiman, N., Henry, T., Dauerman, Udelson, J.E., Gervino, E.V., Pike, M., Whitehouse, M.J., Moon, T., & Chronos, N.A. (2002). Pharmacological Treatment of Coronary Artery Disease With Recombinant Fibroblast Growth Factor-2. Circulation, 105(7), 788-793. 10.1161/hc0802.104407
120. Ivan, D.C., Berve, K.C., Walthert, S., Monaco, G., Borst, K., Bouillet, E., Ferreira, F., Lee, H., Steudler, J., Buch, T., Prinz, M., Engelhardt, B., & Locatelli, G. (2023). Insulin-Like Growth Factor-1 Receptor Controls the Function of CNS-Resident Macrophages and Their Contribution to Neuroinflammation. Acta neuropathologica communications, 11(1), 35. 10.1186/s40478-023-01535-8
121. Yamakawa S, & Hayashida K. (2019). Advances in Surgical Applications of Growth Factors for Wound Healing. Burns & trauma, 7(1), 10. 10.1186/s41038-019-0148-1
122. Park, J.W., Hwang, S.R., & Yoon, I.S. (2017). Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules, 22(8), 1259. 10.3390/molecules22081259
123. Ingle, R.G., & Fang, W.J. (2023). An Overview of the Stability and Delivery Challenges of Commercial Nucleic Acid Therapeutics. Pharmaceutics, 15(4), 1158. 10.3390/pharmaceutics15041158
124. Baker, D.E., & Demaris, K. (2016). Defibrotide. Hospital pharmacy, 51(10), 847-854. 10.1310/hpj5110-847
125. Chambergo-Michilot, D., Alur, A., Kulkarni, S., & Agarwala, A. Mipomersen in Familial Hypercholesterolemia: An Update on Health-Related Quality of Life and Patient-Reported Outcomes. Vascular health and risk management, 18, 73-80. 10.2147/VHRM.S191965
126. Vinores, S.A. (2006). Pegaptanib in the treatment of wet, age-related macular degeneration. International journal of nanomedicine, 1(3), 263-268. 17717967
127. Wilton-Clark, H., & Yokota, T. (2021). Casimersen for Duchenne Muscular Dystrophy. Drugs today (Barc), 57(12), 707-717. 10.1358/dot.2021.57.12.3352740
128. Robinson, C., Pham, C., Zamarripa, A.M., Dugay, C.S., Lee, C.A., Berger, A.A., Landman, A., Cornett, E.M., Kassem, H., Kaye, A.D., Urits, I., Viswanath, O., & Ganti, L. (2022). Inotersen to Treat Polyneuropathy Associated with Hereditary Transthyretin (hATTR) Amyloidosis. Health psychology research, 10(5), 67910. 10.52965/001c.67910
129. Errico, F., Marino, C., Grimaldi, M., Nuzzo, T., Bassareo, V., Valsecchi, V., Panicucci, C., Schiavi, E.D., Mazza, T., Bruno, C., D’Amico, A., Carta, M., D’Ursi, A.M., Bertini, E., Pellizzoni, L., & Usiello, A. (2022). Nusinersen Induces Disease-Severity-Specific Neurometabolic Effects in Spinal Muscular Atrophy. Biomolecules, 12(10), 1431. 10.3390/biom12101431
130. Witztum, J.L., Gaudet, D., Freedman, S.D., Alexander, V.J., Digenio, A., Williams, K.R., Yang, Q., Hughes, S.G., Geary, R.S., Arca, M., Stroes, E.S.G., Bergeron, J., Soran, H., Civeira, F., Hemphill, L., Tsimikas, S., Blom, D.J., O’Dea, L., & Bruckert, E. (2019). Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. The new England journal of medicine, 381(6), 531-542. 10.1056/NEJMoa1715944
131. Yasuda, M., & Keel, S., & Balwani, M. (2023). RNA Interference Therapy in Acute Hepatic Porphyrias. Blood, 142(19), 1589-1599. 10.1182/blood.2022018662
132. Cupido, A.J., & Kastelein, J.J.P. (2020). Inclisiran for the Treatment of Hypercholesterolaemia: Implications and Unanswered Questions from the ORION Trials. Cardiovascular research, 116(11), e136-e139. 10.1093/cvr/cvaa212
133. Scott, L.J., & Keam, S.J. (2021). Lumasiran: First Approval. Drugs, 81(2), 277-282. 10.1007/s40265-020-01463-0
134. Adams, D., Tournev, I.L., Taylor, M.S., Coelho, T., Planté-Bordeneuve, V., Berk, J.L., González-Duarte, A., Gillmore, J.D., Low, S.-C., Sekijima, Y., Obici, L., Chen, C., Badri, P., Arum, S.M., Vest, J., & Polydefkis, M. Efficacy and Safety of Vutrisiran for Patients With Hereditary Transthyretin-Mediated Amyloidosis With Polyneuropathy: A Randomized Clinical Trial. Amyloid, 30(1), 1-9. 10.1080/13506129.2022.2091985
135. Weng, Y., Li, C., Yang, T., Hu, B., Zhang, M., Guo, S., Xiao, H., Liang, X.-J., & Huang, Y. (2020). The Challenge and Prospect of mRNA Therapeutics Landscape. Biotechnology advances, 40, 107534. 10.1016/j.biotechadv.2020.107534
136. Yang, Y., Qin, Z., Zeng, W., Yang, T., Cao, Y., Mei, C., & Kuang, Y. (2016). Toxicity Assessment of Nanoparticles in Various Systems and Organs. Nanotechnology reviews, 6(3), 279-289. 10.1515/ntrev-2016-0047
137. Thi, T.T.H., Suys, E.J.A., Lee, J.S., Nguyen, D.H., Park, K.D., & Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines (Basel), 9(4), 359. 10.3390/vaccines9040359
138. Wang, J., Zhang, Y., Lu, Q., Xing, D., & Zhang, R. (2021). Exploring Carbohydrates for Therapeutics: A Review on Future Directions. Frontiers in pharmacology, 12, 756724. 10.3389/fphar.2021.756724
139. Tovar, A.M.F., Santos, G.R.C., Capillé, N.V., Piquet, A.A., Glauser, B.F., Pereira, M.S., Vilanova, E., & Mourão, P.A.S. (2016). Structural and Haemostatic Features of Pharmaceutical Heparins from Different Animal Sources: Challenges to Define Thresholds Separating Distinct Drugs. Scientific reports, 6(1), 35619. 10.1038/srep35619
140. Bae, S.H., Kim, M.R. (2020). Subtype Classification of Functional Constipation in Children: Polyethylene Glycol Versus Lactulose. Pediatrics international, 62(7), 816-819. 10.1111/ped.14235
141. Ben-Haim, S., & Ell, P. (2009). 18F-FDG PET and PET/CT in the Evaluation of Cancer Treatment Response. Journal of nuclear medicine, 50(1), 88-99. 10.2967/jnumed.108.054205
142. Bensinger, S.J., & Christofk, H.R. (2012). New Aspects of the Warburg Effect in Cancer Cell Biology. Seminars in cell & developmental biology, 23(4), 352-361. 10.1016/j.semcdb.2012.02.003
143. Lenci, E., & Trabocchi, A. (2020). Peptidomimetic Toolbox for Drug Discovery. Chemical society reviews, 49(11), 3262-3277. 10.1039/D0CS00102C
144. Mettu, R., Chen, C.-Y., & Wu, C.-Y. (2020). Synthetic Carbohydrate-Based Vaccines: Challenges and Opportunities. Journal of biomedical science, 27(1), 9. 10.1186/s12929-019-0591-0
145. Delorme, V., Lichon, L., Mahindad, H., Hunger, S., Laroui, N., Daurat, M., Godefroy, A., Coudane, J., Gary-Bobo, M., & Berghe, H.V.D. (2020). Reverse Poly(ε-caprolactone)-g-dextran Graft Copolymers. Nano-Carriers for Intracellular Uptake of Anticancer Drugs. Carbohydrate polymers, 232, 115764. 10.1016/j.carbpol.2019.115764
146. Yang, O., Qadan, M., & Ierapetritou, M. (2019). Economic Analysis of Batch and Continuous Biopharmaceutical Antibody Production: A Review. Journal of pharmaceutical innovation, 14, 1-19. 10.1007/s12247-018-09370-4
147. Van Norman, G.A. (2019). Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC: basic to translational science, 4(7), 845-854. 10.1016/j.jacbts.2019.10.008
