| Original Article, Biomed Biopharm Res., 2023; 20(2):3-13 doi: 10.19277/bbr.20.2.317; Bilingual PDF [+]; html in Portuguese [PT] |
Effects on expression of B lymphocyte transmembrane ligand genes after treatment with Rose extract
Mark Christopher Arokiaraj 1 ![]() ✉️ & Eric Menesson 2
✉️ & Eric Menesson 2
1 - Pondicherry Institute of Medical Sciences, Kalapet, Puducherry, India
2 - Tebubio,Le Perray en Yvelines, France
Abstract
The goal was to evaluate the role of red rose extract (Pierre de Ronsard) on the human B lymphocytes gene CD20, CD30, CD40, and CCR5 expression. Red rose extract was prepared at the dilution of 0.0075% (v/v) and stored until use at -20°C. Cell treatment was performed at 37°C on human B cells. Cells were plated in 6 well plates at 1.5x106 cells per well and stored at -80°C and total RNA extracted. RTq-PCR was performed according to Genecopoeia. The cycle threshold method (ΔΔCt) was used for data analysis. The comparative Ct method quantification (2^-ΔCt) and fold change for CD20, CD30, CD40 and CCR5 were - 5.65E+01, 4.80E-01, N/A, 2.47E-01; and 0.954,0.377, N/A and 0.577, respectively. The amount of total RNA extracted from about 4.5x10⁶ cells was low and did not allow us to measure the RNA profile. With the exception of CD40, all other genes were expressed and well-measured in both B cell samples by qRT-PCR. The expression of CD30 and CCR5 genes were decreased in B lymphocytes in vitro with the rose extract treatment. More studies are needed to further study these effects and potential.
Keywords: Rose extract, B lymphocytes, CD30, CCR5
To Cite: Arokiaraj, M.C. & Menesson, E. (2023) Effects on expression of B lymphocyte transmembrane ligand genes after treatment with Rose extract. Biomedical and Biopharmaceutical Research, 20(2), 2-13.
Author correspondence:
Received: 18/05/2023; Accepted: 30/09/2023
Introduction
B lymphocytes are known for their pivotal role in modulating immunity. They play a significant role in both innate and adaptive immunity which might be modified by these cells (1-4). Autoimmune disorders are modulated by B cells (2). B cells have a role in antibody synthesis, antigen presentation, cytokine, and chemokine synthesis (3). Cross-reactive antibodies synthesised by B cells help to regulate B cell memory and other immune-modulatory functions (4). The idiotype 9G4 (9G4+) antibody produced in B cells’ function as an interface between innate and adaptive immunity (1). B cell depletion therapies have been used for the treatment of autoimmune disorders where CD20, CD30, CD 40, and CCR5 ligands are expressed on the surface of the B cells (5). These ligands are plasma membrane phosphoproteins and have deeper connections in the cells while their expression is modified in various diseases and conditions based on the cell and extracellular signals. CD 20 is expressed in a wide range of tumours and autoimmune disorder regulation (6-9). CD30 expression is found on Hodgkin and Reed-Sternberg cells, anaplastic large-cell lymphoma cells, and activated B or T lymphocytes. CD30 has been shown to be a transmembrane receptor that is significantly homologous to the tumour necrosis factor receptor (TNFR) family (10-11). CD40 is expressed primarily by activated T cells, as well as activated B cells and platelets, and is also induced on monocytic cells, natural killer cells, mast cells, and basophils under inflammatory conditions (12-15). The CCRF motif is multifunctional and plays a role in immune regulation and neointimal proliferation (16-17).
There are currently many drugs which modulate the effect of these ligands in various diseases, however, these medications are also associated with various side effects which would limit their use (18-25). The CD 20, CD 30, CD 40 and CCR5 are major transmembrane ligands associated with some important diseases. In previous studies, we observed the immunomodulatory effects of treatment with rose extract by the reduction of cytokines in endothelial cells (26) and the increase of certain cytokines by the T cells (27), as well as the increase in soluble CCR5 and prevention of hypoxic reduction of soluble CXCR4 of the endothelial cells (17). In this study, the rose extract was used to evaluate its impact on B cells. The regulation of these ligands on the B cell surface will provide more insights into the immune modulation of the rose extract.
Methods
The preparation of the rose extract was previously described (26). The prepared extract was stored at -20 ºC prior to use. The Rosa Rosaceae (Pierre de Ronsard) flower was chosen from the garden of the Tebu Bio Institute in Le Perray en Yvelines (France). In our previous study, we have shown that the rose extract at concentrations of 0.5% or lower is not associated with cell lysis (26).
Six vials of B cells, obtained from human peripheral mononuclear cells (PBMC) using positive immunomagnetic selection directly against CD19 and cryopreserved immediately after isolation, were thawed, pooled, and plated in each well of a 6-well plate at 1.5x10⁶ cells per well. After 24 h, the cells of three wells were treated for 24 h with 0.0075% (v/v) rose extract prepared during the project POMC-032018. In the remaining three wells, the B cells were untreated. After 24 h incubation, the treated and untreated cells were collected and washed with PBS twice. They were then centrifuged and stored at -80°C as cell pellets until RNA extraction. We chose 0.075% (v/v) as a safe and effective concentration for testing on the B cells in this study, since in our previous study the cytotoxic concentration on the endothelial cells was 0.5% or above and the effects of the rose extract treatment were detected to a concentration of 0.001%.
The materials used in the study were human peripheral blood B cells (CellApplications, ref. 6904-20a, lot 3342), blood cell growth medium kit (Cell Applications, ref. 615K-250), NucleoSpin RNA plus kit (Macherey-Nagel, ref. 740984.10) and BlazeTaq One-Step SYBR Green RT-qPCR Kit (Genecopoeia, ref. QP081). All-in-OneTM qPCR human Primer from Genecopoeia for HPRT1, NM_000194.2 (Ref. HQP009026), UBE2D2, NM_181838.1 (Ref. HQP018366), CD20/MS4A1, NM_152867.2 (Ref. HQP118276), CD30/TNFRSF8, NM_001281430.2 (Ref. HQP059085), CD40/TNFRSF5, NM_001322422.1 (Ref. HQP116918), CCR5, NM_000579.3 (Ref. HQP002210) were also used in the study.
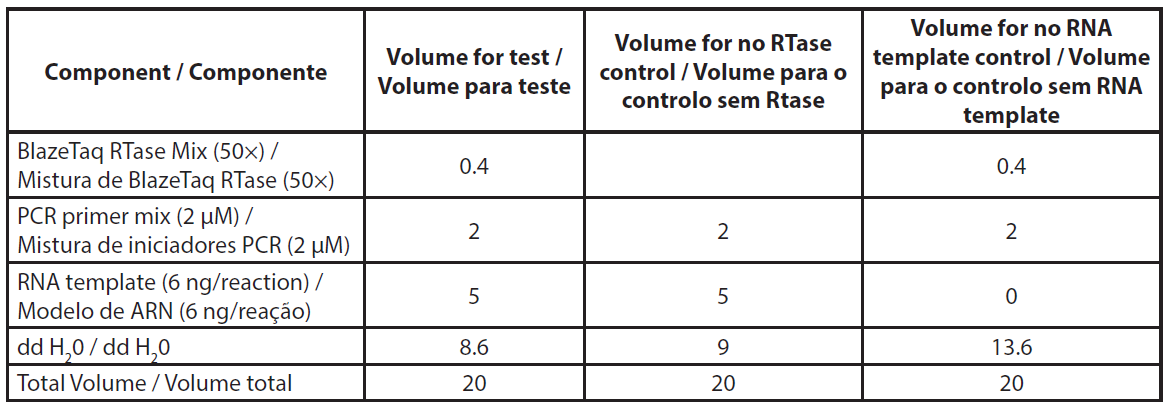
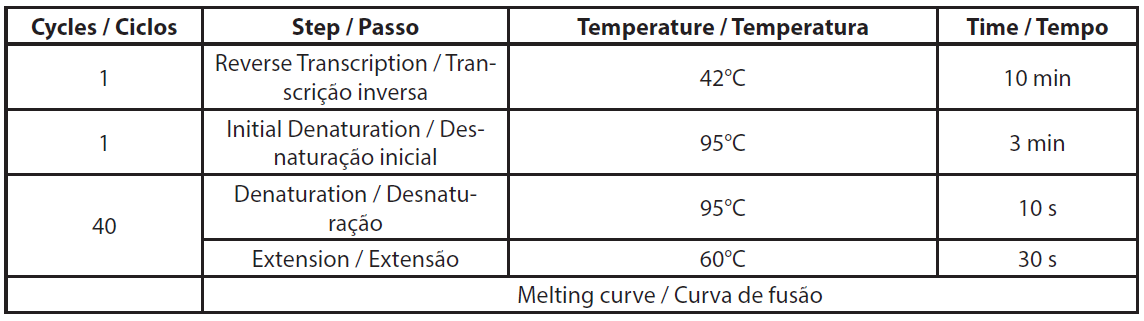
Total RNA was extracted using NucleoSpin RNA plus kit (Macherey-Nagel, ref. 740984.10) according to the manufacturer’s protocol, including the DNase treatment. Extracted RNA was stored at -80°C for qRT-PCR assay. The qRT-PCR was conducted according to the Genecopoeia’s instructions. The BlazeTaq™ One-Step SYBR® Green RT-qPCR kit is designed to perform RT and real-time PCR in one step. The qRT-PCR was performed using 6 ng of total RNA. Two controls were made per sample, without RTase and without RNA template (NTC). The required volumes for qRT-PCR are presented in Table 1. The components mix was distributed in a PCR plate. Primer mix and RNA template were added to corresponding wells. PCR was performed using Eppendorf Mastercycler RealPlex 2S by following the PCR program presented in Table 2.
| Table 1. Description of qRT-PCR reaction mix. |
 |
| Table 2. Details of qRT-PCR program. |
 |
We evaluated the delta Ct (∆Ct) value for each sample by subtracting the Ct value of the reference gene from the Ct value of the target gene. This gives the difference in gene expression levels between the target gene and reference gene in each sample. The mean delta Ct value was calculated for each group with or without treatment with rose extract. The delta-delta Ct method, also known as the 2–∆∆Ct method, is a simple formula used in order to calculate the relative fold gene expression of samples when performing real-time polymerase chain reaction or qPCR.
The ΔΔCt (cycle threshold) method was used for data analysis. In separate reactions, the Ct value was determined for each replicate of the housekeeping gene HPRT1 and UBE2D2 (HKG) and gene of interest (GOI) in both samples. For each sample, the difference between the Ct average of GOI replicate and the Ct average for the HKG was calculated (ΔCt). The normalized GOI gene expression was then determined as 2(–ΔΔCt).
Absorbance spectra values were measured using a NanoVue™ spectrophotometer (GE Healthcare, Piscataway, NJ, USA).
Results
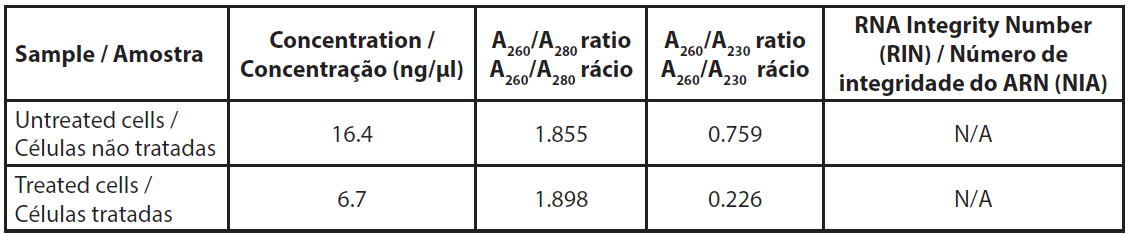
The amount of total RNA extracted from about 4.5x106 cells was low and did not allow us to measure the RNA profile (Table 3). The A260/A230 ratios were very low, indicating a relatively high proportion of salts in the samples, however, this was due to the very low amount of RNA rather than an unexpected quantity of salts after extraction.
| Table 3. Absorbance of extracted RNAs. |
 |
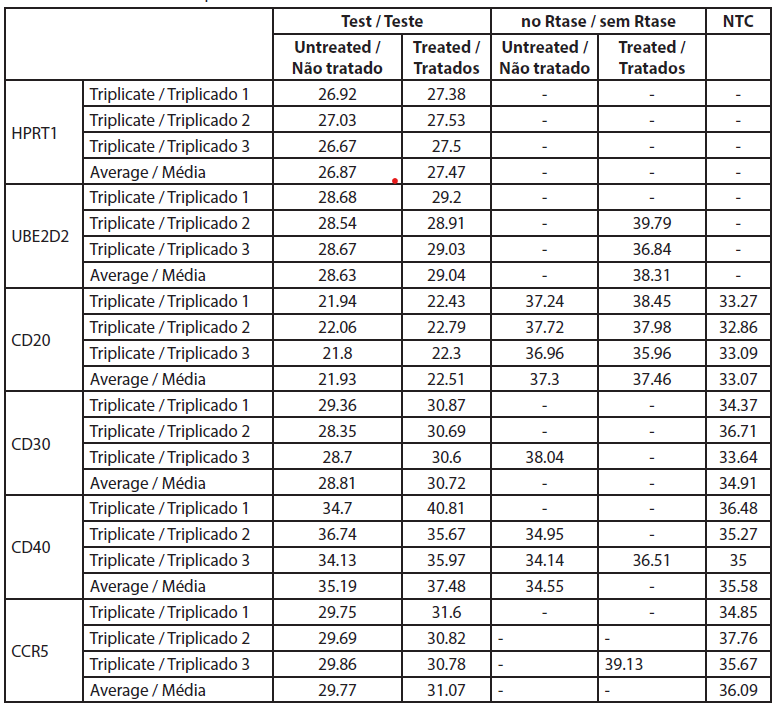
The fold-change is the normalized gene expression in the treated sample divided the normalized gene expression in untreated cells. Raw data are listed in Table 4 and the normalized gene expressions and fold changes are shown in Table 5.
| Table 4. Ct values from the qRT-PCR. |
 |
| Table 5. Gene expression fold change between treated and untreated B cells. |
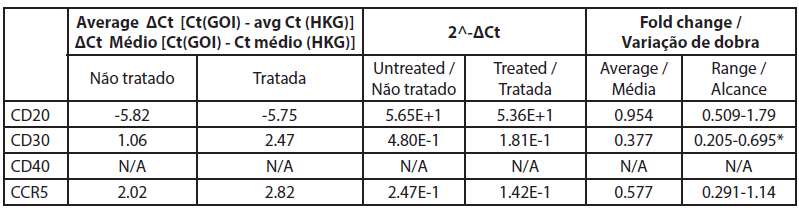 |
The analysis of gene expression by qRT-PCR showed that CD40 was not expressed in untreated cells and cells treated with rose extract with the Ct values over 32 (Table 5). The CD20 levels were not significantly altered by the rose extract treatment in this study. All other genes were expressed and were well measured in both B cell treated and untreated cell samples. The CT values of CD20 genes before and after rose extract treatment were -5.82 and -5.75, respectively, and the average fold change was 0.95 (0.51-1.79), which was not significant.
In this study, the expression of CD30 on the B cells was reduced after treatment with rose extract (0.0075%). It was reduced to a 0.377-fold change compared to untreated cells, and the results were consistent (0.205 to 0.695). The expression of CCR5 was also reduced by a -0.577-fold change, but the reduction was inconsistent as it crossed the non-specific mark (0.29 to 1.14).
Discussion
This study suggests that treatment with rose extract results in a reduction in expression of CD30 in B lymphocytes and a tendency to reduce CCR5 gene expression. There were no changes in the expression of the CD20 gene after rose extract treatment, and CD40 was not expressed in treated or untreated B cells.
A clinical benefit would be the reduction of CD30 expression in cancerous cells. CD30 is an active marker in major tumours such as Hodgkins lymphoma, anaplastic large cell lymphoma, peripheral T cell lymphoma, and adult T cell lymphoma/leukemia, as well as in non lymphomatous malignancies (28,29). It also plays a role in angiogenic cancers such as angiosarcomas, epithelioid haemangioendothelioma, or Kaposi’s sarcoma (30). CD30 inhibition is also associated with the treatment of primary effusion lymphoma (31). CD30 is also an active marker in autoimmune disorders but its role in this treatment is not clearly established. Targeting CD 30-30L has been used in the treatment of autoimmune and inflammatory disorders (32). Remission of rheumatoid arthritis has been observed in the treatment with brentuximab vedotin, and anti-CD30 agent (33). Inhibition of CD30 in T cells is also associated with reduced graft vs host disease (34) and immune complex-mediated glomerulonephritis (35). CD30-30L interaction inhibition has been experimentally associated with reduced atherosclerosis (36). Soluble CD30 is reduced in cases with stable coronary artery disease (37). CD30 expression is increased in patients with chronic obstructive pulmonary disease and in vascular remodelling (38).
Similarly, the efficacy of the rose extract in acquired immunodeficiency disorder treatment by the reduction in CCR5 on the T cell surface needs to be studied in retrovirus-infected cells. In our previous study, we have shown the effects in the reduction of soluble CCR5 in endothelial cells treated with rose extract (17). CCR5 has been shown to have a role in the regulation of vascular, neurological, and signalling in autoimmune disorders (39), as well as being involved in learning, memory, and cognitive functions (40).
Rose extract has been known for its anti-inflammatory, antioxidant, antidiabetic, and antidepressant effects (41, 42) also including a certain antibacterial activity (41-43). In previous studies we also have detected some anti-inflammatory effects of rose extract on T cells. The present study shows that the beneficial effects might be related to some extent with a reduction in CD30 levels, and CCR5 levels on the B cell surface.
Some limitations should be considered in this study. It was done in vitro, and certainly wider sample size dimensions are required for a full in vitro as in vivo concept evaluation. This study was very focused in certain cell ligands at the cell surface. Further evaluation is required with the detailed study on cytokines for more information on the B cell function after 0.0075% rose extract treatment. Finally, the active molecules involved in observed effects of the red rose extract need to be studied in detail by phytochemical analysis.
Conclusions
The treatment of B lymphocytes with rose extract at 0.0075% (v/v) did not modify the expression of CD20. However, the expression of CD30 and CCR5 seemed to be decreased by the treatment. The range of the fold change showed that the result of CD30 expression was more accurate than for CCR5. The reduction of CD30 and CCR5 expression has potential for clinical applications which need to be studied in detail in in vitro and in vivo pathological models.
Funding
None
Authors Contributions Statement
MCA conceived the idea and method, designed the study, interpreted results and wrote the paper. EM prepared the methods protocol, performed the experiments and derived the results.
Conflict of Interests
The authors declare there are no financial and/or personal relationships that could present a potential conflict of interests.
References
1. Milner, E. C., Anolik, J., Cappione, A., & Sanz, I. (2005). Human innate B cells: a link between host defense and autoimmunity?. Springer seminars in immunopathology, 26(4), 433–452. https://doi.org/10.1007/s00281-004-0188-9
2. Tsay, G. J., & Zouali, M. (2018). The Interplay Between Innate-Like B Cells and Other Cell Types in Autoimmunity. Frontiers in immunology, 9, 1064. https://doi.org/10.3389/fimmu.2018.01064
3. Zhang X. (2013). Regulatory functions of innate-like B cells. Cellular & molecular immunology, 10(2), 113–121. https://doi.org/10.1038/cmi.2012.63
4. Grasseau, A., Boudigou, M., Le Pottier, L., Chriti, N., Cornec, D., Pers, J. O., Renaudineau, Y., & Hillion, S. (2020). Innate B Cells: the Archetype of Protective Immune Cells. Clinical reviews in allergy & immunology, 58(1), 92–106. https://doi.org/10.1007/s12016-019-08748-7
5. Lee, D. S. W., Rojas, O. L., & Gommerman, J. L. (2021). B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nature reviews. Drug discovery, 20(3), 179–199. https://doi.org/10.1038/s41573-020-00092-2
6. Pavlasova, G., & Mraz, M. (2020). The regulation and function of CD20: an "enigma" of B-cell biology and targeted therapy. Haematologica, 105(6), 1494–1506. https://doi.org/10.3324/haematol.
7. Kläsener, K., Jellusova, J., Andrieux, G., Salzer, U., Böhler, C., Steiner, S. N., Albinus, J. B., Cavallari, M., Süß, B., Voll, R. E., Boerries, M., Wollscheid, B., & Reth, M. (2021). CD20 as a gatekeeper of the resting state of human B cells. Proceedings of the National Academy of Sciences of the United States of America, 118(7), e2021342118. https://doi.org/10.1073/pnas.2021342118
8. CD30 [Internet]. Pathology Outlines - PathologyOutlines.com. Available from: https://www.pathologyoutlines.com/topic/cdmarkerscd30.html
9. Michot, J. M., Buet-Elfassy, A., Annereau, M., Lazarovici, J., Danu, A., Sarkozy, C., Chahine, C., Bigenwald, C., Bosq, J., Rossignol, J., Romano-Martin, P., Baldini, C., Ghez, D., Dartigues, P., Massard, C., & Ribrag, V. (2021). Clinical significance of the loss of CD20 antigen on tumor cells in patients with relapsed or refractory follicular lymphoma. Cancer drug resistance (Alhambra, Calif.), 4(3), 710–718. https://doi.org/10.20517/cdr.2020.109
10. de Bruin, P. C., Gruss, H. J., van der Valk, P., Willemze, R., & Meijer, C. J. (1995). CD30 expression in normal and neoplastic lymphoid tissue: biological aspects and clinical implications. Leukemia, 9(10), 1620–1627.
11. van der Weyden, C. A., Pileri, S. A., Feldman, A. L., Whisstock, J., & Prince, H. M. (2017). Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood cancer journal, 7(9), e603. https://doi.org/10.1038/bcj.2017.85
12. Funakoshi, S., Longo, D. L., Beckwith, M., Conley, D. K., Tsarfaty, G., Tsarfaty, I., Armitage, R. J., Fanslow, W. C., Spriggs, M. K., & Murphy, W. J. (1994). Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood, 83(10), 2787–2794.
13. Linderoth, J., Ehinger, M., Jerkeman, M., Bendahl, P. O., Akerman, M., Berglund, M., Enblad, G., Erlanson, M., Roos, G., & Cavallin-Ståhl, E. (2007). CD40 expression identifies a prognostically favourable subgroup of diffuse large B-cell lymphoma. Leukemia & lymphoma, 48(9), 1774–1779. https://doi.org/10.1080/10428190701494520
14. Feng, Z., & Wang, J. (2021). Soluble CD40 ligand inhibits the growth of non-Hodgkin's lymphoma cells through the JNK signaling pathway. Oncology letters, 21(1), 56. https://doi.org/10.3892/ol.2020.12318
15. Karnell, J. L., Rieder, S. A., Ettinger, R., & Kolbeck, R. (2019). Targeting the CD40-CD40L pathway in autoimmune diseases: Humoral immunity and beyond. Advanced drug delivery reviews, 141, 92–103. https://doi.org/10.1016/j.addr.2018.12.005
16. Oppermann M. (2004). Chemokine receptor CCR5: insights into structure, function, and regulation. Cellular signalling, 16(11), 1201–1210. https://doi.org/10.1016/j.cellsig.2004.04.007
17. Arokiaraj, M., & Menesson, E. (2021). Effect of rose extract treatment on soluble CCR5 and CXCR4 secretion by the endothelial cells in vitro.Biomedical Research and Therapy, 8(5), 4333-4344. https://doi.org/10.15419/bmrat.v8i5.672
18. Hansel, T. T., Kropshofer, H., Singer, T., Mitchell, J. A., & George, A. J. (2010). The safety and side effects of monoclonal antibodies. Nature reviews. Drug discovery, 9(4), 325–338. https://doi.org/10.1038/nrd3003
19. Du, F. H., Mills, E. A., & Mao-Draayer, Y. (2017). Next-generation anti-CD20 monoclonal antibodies in autoimmune disease treatment. Auto- immunity highlights, 8(1), 12. https://doi.org/10.1007/s13317-017-0100-y
20. Payandeh, Z., Bahrami, A. A., Hoseinpoor, R., Mortazavi, Y., Rajabibazl, M., Rahimpour, A., Taromchi, A. H., & Khalil, S. (2019). The applications of anti-CD20 antibodies to treat various B cells disorders. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 109, 2415–2426. https://doi.org/10.1016/j.biopha.2018.11.121
21. van Vollenhoven, R. F., Emery, P., Bingham, C. O., 3rd, Keystone, E. C., Fleischmann, R. M., Furst, D. E., Tyson, N., Collinson, N., & Lehane, P. B. (2013). Long-term safety of rituximab in rheumatoid arthritis: 9.5-year follow-up of the global clinical trial programme with a focus on adverse events of interest in RA patients. Annals of the rheumatic diseases, 72(9), 1496–1502. https://doi.org/10.1136/annrheumdis-2012-201956
22. Yi, J. H., Kim, S. J., & Kim, W. S. (2017). Brentuximab vedotin: clinical updates and practical guidance. Blood research, 52(4), 243–253. https://doi.org/10.5045/br.2017.52.4.243
23. Wang, Y., Nowakowski, G. S., Wang, M. L., & Ansell, S. M. (2018). Advances in CD30- and PD-1-targeted therapies for classical Hodgkin lymphoma. Journal of hematology & oncology, 11(1), 57. https://doi.org/10.1186/s13045-018-0601-9
24. Ramos, C. A., Grover, N. S., Beaven, A. W., Lulla, P. D., Wu, M. F., Ivanova, A., Wang, T., Shea, T. C., Rooney, C. M., Dittus, C., Park, S. I., Gee, A. P., Eldridge, P. W., McKay, K. L., Mehta, B., Cheng, C. J., Buchanan, F. B., Grilley, B. J., Morrison, K., Brenner, M. K., … Savoldo, B. (2020). Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 38(32), 3794–3804. https://doi.org/10.1200/JCO.20.01342
25. Muta, H., & Podack, E. R. (2013). CD30: from basic research to cancer therapy. Immunologic research, 57(1-3), 151–158. https://doi.org/10.1007/s12026-013-8464-1
26. Arokiaraj, M. C., & Menesson, E. (2020). Novel anti-inflammatory and immunomodulation effects of rose on the endothelium in normal and hypoxic invitro conditions. Angiologia E Cirurgia Vascular, 15(4), 238–248. https://doi.org/10.48750/acv.221
27. Arokiaraj, M. C., & Menesson, E. (2022). Cytokine Response of CD4+ T-Lymphocytes with Red Rose (Rosa Rosaceae – Pierre de Ronsard) Extracts by in Vitro Evaluation. Galician Medical Journal, 29(1), E202215. https://doi.org/10.21802/gmj.2022.1.5
28. Nakashima, M, & Uchimaru, K. (2023) CD30 Expression and Its Functions during the Disease Progression of Adult T-Cell Leukemia/Lymphoma. International Journal of Molecular Sciences, 24(10):8731. https://doi.org/10.3390/ijms2410873
29. Sharman, J.P., Goldschmidt, J. H., Burke, J. M., Hellerstedt, B. A., McIntyre, K. et al. (2012) CD30 expression in nonlymphomatous malignancies. Journal of Clinical Oncology30:15_suppl, 3069. DOI: 10.1200/jco.2012.30.15_suppl.3069
30. Alimchandani, M., Wang, Z. F., & Miettinen, M. (2014). CD30 expression in malignant vascular tumors and its diagnostic and clinical implications: a study of 146 cases. Applied immunohistochemistry & molecular morphology : AIMM, 22(5), 358–362. https://doi.org/10.1097/PAI.0000000000000048
31. Bhatt, S., Ashlock, B. M., Natkunam, Y., Sujoy, V., Chapman, J. R., Ramos, J. C., Mesri, E. A., & Lossos, I. S. (2013). CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood, 122(7), 1233–1242. https://doi.org/10.1182/blood-2013-01-481713
32. Oflazoglu, E., Grewal, I. S., & Gerber, H. (2009). Targeting CD30/CD30L in oncology and autoimmune and inflammatory diseases. Advances in experimental medicine and biology, 647, 174–185. https://doi.org/10.1007/978-0-387-89520-8_12
33. Vachhani, P., Bose, N., Brodeur, J. P., Holkova, B., & Bose, P. (2014). Remission of rheumatoid arthritis on brentuximab vedotin. Rheumatology (Oxford, England), 53(12), 2314–2315. https://doi.org/10.1093/rheumatology/keu374
34. Chen, Y. B., McDonough, S., Hasserjian, R., Chen, H., Coughlin, E., Illiano, C., Park, I. S., Jagasia, M., Spitzer, T. R., Cutler, C. S., Soiffer, R. J., & Ritz, J. (2012). Expression of CD30 in patients with acute graft-versus-host disease. Blood, 120(3), 691–696. https://doi.org/10.1182/blood-2012-03-415422
35. Artinger, K., Kirsch, A. H., Mooslechner, A. A., Cooper, D. J., Aringer, I., Schuller, M., Schabhüttl, C., Klötzer, K. A., Schweighofer, K., Eller, P., Yagita, H., Illert, A. L., Rosenkranz, A. R., Lane, P. J., & Eller, K. (2021). Blockade of tumor necrosis factor superfamily members CD30 and OX40 abrogates disease activity in murine immune-mediated glomerulonephritis. Kidney international, 100(2), 336–348. https://doi.org/10.1016/j.kint.2021.02.039
36. Foks, A. C., Bot, I., Frodermann, V., de Jager, S. C., Ter Borg, M., van Santbrink, P. J., Yagita, H., Kuiper, J., & van Puijvelde, G. H. (2012). Interference of the CD30-CD30L pathway reduces atherosclerosis development. Arteriosclerosis, thrombosis, and vascular biology, 32(12), 2862–2868. https://doi.org/10.1161/ATVBAHA.112.300509
37. Mahmoudi, M. J., Hedayat, M., Rezaei, N., Saboor-Yaraghi, A. A., & Mahmoudi, M. (2011). In vitro soluble CD30 levels in patients with chronic stable coronary artery disease. Iranian journal of allergy, asthma, and immunology, 10(4), 237–242.
38. Luo, L., Liu, Y., Chen, D., Chen, F., Lan, H. B., & Xie, C. (2018). CD30 Is Highly Expressed in Chronic Obstructive Pulmonary Disease and Induces the Pulmonary Vascular Remodeling. BioMed research international, 2018, 3261436. https://doi.org/10.1155/2018/3261436
39. Jones, K. L., Maguire, J. J., & Davenport, A. P. (2011). Chemokine receptor CCR5: from AIDS to atherosclerosis. British journal of pharmacology, 162(7), 1453–1469. https://doi.org/10.1111/j.1476-5381.2010.01147.x
40. Necula, D., Riviere-Cazaux, C., Shen, Y., & Zhou, M. (2021). Insight into the roles of CCR5 in learning and memory in normal and disordered states. Brain, behavior, and immunity, 92, 1–9. https://doi.org/10.1016/j.bbi.2020.11.037
41. Mahboubi M. (2015). Rosa damascena as holy ancient herb with novel applications. Journal of traditional and complementary medicine, 6(1), 10–16. https://doi.org/10.1016/j.jtcme.2015.09.005
42. Lee, M. H., Nam, T. G., Lee, I., Shin, E. J., Han, A. R., Lee, P., Lee, S. Y., & Lim, T. G. (2018). Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food science & nutrition, 6(8), 2560–2567. https://doi.org/10.1002/fsn3.870
43. Safdar Y., Malik T. (2020) Antibacterial activity of rose extract. Open Access journal of complementary and alternative medicine, 2(4), 194-201. DOI: 10.32474/OAJCAM.2020.02.000144
